Kinetic analysis of paramyxovirus-sialoglycan receptor interactions reveals virion motility
- PMID: 36972304
- PMCID: PMC10079232
- DOI: 10.1371/journal.ppat.1011273
Kinetic analysis of paramyxovirus-sialoglycan receptor interactions reveals virion motility
Abstract
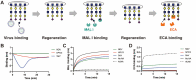
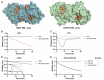
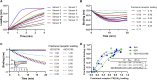
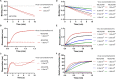
Many viruses initiate infection by binding to sialoglycan receptors at the cell surface. Binding to such receptors comes at a cost, however, as the sheer abundance of sialoglycans e.g. in mucus, may immobilize virions to non-functional decoy receptors. As a solution, sialoglycan-binding as well as sialoglycan-cleavage activities are often present in these viruses, which for paramyxoviruses are combined in the hemagglutinin-neuraminidase (HN) protein. The dynamic interactions of sialoglycan-binding paramyxoviruses with their receptors are thought to be key determinants of species tropism, replication and pathogenesis. Here we used biolayer interferometry to perform kinetic analyses of receptor interactions of animal and human paramyxoviruses (Newcastle disease virus, Sendai virus, and human parainfluenza virus 3). We show that these viruses display strikingly different receptor interaction dynamics, which correlated with their receptor-binding and -cleavage activities and the presence of a second sialic acid binding site. Virion binding was followed by sialidase-driven release, during which virions cleaved sialoglycans until a virus-specific density was reached, which was largely independent of virion concentration. Sialidase-driven virion release was furthermore shown to be a cooperative process and to be affected by pH. We propose that paramyxoviruses display sialidase-driven virion motility on a receptor-coated surface, until a threshold receptor density is reached at which virions start to dissociate. Similar motility has previously been observed for influenza viruses and is likely to also apply to sialoglycan-interacting embecoviruses. Analysis of the balance between receptor-binding and -cleavage increases our understanding of host species tropism determinants and zoonotic potential of viruses.
Copyright: © 2023 Wu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

