Pathology and Mineralogy of the Pneumoconioses
- PMID: 36972614
- PMCID: PMC11617341
- DOI: 10.1055/s-0043-1764406
Pathology and Mineralogy of the Pneumoconioses
Abstract
Pneumoconioses represent the spectrum of lung diseases caused by inhalation of respirable particulate matter small enough (typically <5-µm diameter) to reach the terminal airways and alveoli. Pneumoconioses primarily occur in occupational settings where workers perform demanding and skilled manual labor including mining, construction, stone fabrication, farming, plumbing, electronics manufacturing, shipyards, and more. Most pneumoconioses develop after decades of exposure, though shorter latencies can occur from more intense particulate matter exposures. In this review, we summarize the industrial exposures, pathologic findings, and mineralogic features of various well-characterized pneumoconioses including silicosis, silicatosis, mixed-dust pneumoconiosis, coal workers' pneumoconiosis, asbestosis, chronic beryllium disease, aluminosis, hard metal pneumoconiosis, and some less severe pneumoconioses. We also review a general framework for the diagnostic work-up of pneumoconioses for pulmonologists including obtaining a detailed occupational and environmental exposure history. Many pneumoconioses are irreversible and develop due to excessive cumulative respirable dust inhalation. Accurate diagnosis permits interventions to minimize ongoing fibrogenic dust exposure. A consistent occupational exposure history coupled with typical chest imaging findings is usually sufficient to make a clinical diagnosis without the need for tissue sampling. Lung biopsy may be required when exposure history, imaging, and testing are inconsistent, there are unusual or new exposures, or there is a need to obtain tissue for another indication such as suspected malignancy. Close collaboration and information-sharing with the pathologist prior to biopsy is of great importance for diagnosis, as many occupational lung diseases are missed due to insufficient communication. The pathologist has a broad range of analytic techniques including bright-field microscopy, polarized light microscopy, and special histologic stains that may confirm the diagnosis. Advanced techniques for particle characterization such as scanning electron microscopy/energy dispersive spectroscopy may be available in some centers.
Thieme. All rights reserved.
Conflict of interest statement
C. D. C. receives consulting fees for pathology reviews of black lung cases for coal workers and their families. F. H. Y. G. receives consulting fees for expert opinions on cases of occupational lung disease for both plaintiff and defense parties.
Figures

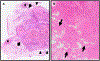
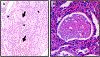


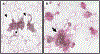




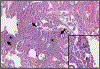

References
-
- Shi P, Xing X, Xi S, et al. Trends in global, regional and national incidence of pneumoconiosis caused by different aetiologies: an analysis from the Global Burden of Disease Study 2017. Occup Environ Med 2020;77(06):407–414 - PubMed
-
- Lax MB, Grant WD, Manetti FA, Klein R. Recognizing occupational disease–taking an effective occupational history. Am Fam Physician 1998;58(04):935–944 - PubMed
-
- Levy BS, Wegman DH. The occupational history in medical practice. What questions to ask and when to ask them. Postgrad Med 1986;79(08):301–311 - PubMed
-
- International Labour Office. Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconiosis, Revised edition 2011. 2011. Accessed September 9, 2022 at: https://www.ilo.org/wcmsp5/groups/public/—ed_protect/—protrav/—safework/...
-
- Tamura T, Suganuma N, Hering KG, et al. Relationships (I) of International Classification of High-resolution Computed Tomography for Occupational and Environmental Respiratory Diseases with the ILO International Classification of Radiographs of Pneumoconioses for parenchymal abnormalities. Ind Health 2015;53(03):260–270 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

