ABCB1 and ABCG2 Regulation at the Blood-Brain Barrier: Potential New Targets to Improve Brain Drug Delivery
- PMID: 36973040
- PMCID: PMC10441638
- DOI: 10.1124/pharmrev.120.000025
ABCB1 and ABCG2 Regulation at the Blood-Brain Barrier: Potential New Targets to Improve Brain Drug Delivery
Abstract
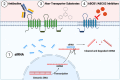
The drug efflux transporters ABCB1 and ABCG2 at the blood-brain barrier limit the delivery of drugs into the brain. Strategies to overcome ABCB1/ABCG2 have been largely unsuccessful, which poses a tremendous clinical problem to successfully treat central nervous system (CNS) diseases. Understanding basic transporter biology, including intracellular regulation mechanisms that control these transporters, is critical to solving this clinical problem.In this comprehensive review, we summarize current knowledge on signaling pathways that regulate ABCB1/ABCG2 at the blood-brain barrier. In Section I, we give a historical overview on blood-brain barrier research and introduce the role that ABCB1 and ABCG2 play in this context. In Section II, we summarize the most important strategies that have been tested to overcome the ABCB1/ABCG2 efflux system at the blood-brain barrier. In Section III, the main component of this review, we provide detailed information on the signaling pathways that have been identified to control ABCB1/ABCG2 at the blood-brain barrier and their potential clinical relevance. This is followed by Section IV, where we explain the clinical implications of ABCB1/ABCG2 regulation in the context of CNS disease. Lastly, in Section V, we conclude by highlighting examples of how transporter regulation could be targeted for therapeutic purposes in the clinic. SIGNIFICANCE STATEMENT: The ABCB1/ABCG2 drug efflux system at the blood-brain barrier poses a significant problem to successful drug delivery to the brain. The article reviews signaling pathways that regulate blood-brain barrier ABCB1/ABCG2 and could potentially be targeted for therapeutic purposes.
Copyright © 2023 by The Author(s).
Figures









References
-
- Abele R, Tampé R (1999) Function of the transport complex TAP in cellular immune recognition. Biochim Biophys Acta 1461:405–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

