Risk of death following COVID-19 vaccination or positive SARS-CoV-2 test in young people in England
- PMID: 36973247
- PMCID: PMC10043280
- DOI: 10.1038/s41467-023-36494-0
Risk of death following COVID-19 vaccination or positive SARS-CoV-2 test in young people in England
Abstract
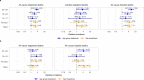
Several studies have reported associations between COVID-19 vaccination and risk of cardiac diseases, especially in young people; the impact on mortality, however, remains unclear. We use national, linked electronic health data in England to assess the impact of COVID-19 vaccination and positive SARS-CoV-2 tests on the risk of cardiac and all-cause mortality in young people (12 to 29 years) using a self-controlled case series design. Here, we show there is no significant increase in cardiac or all-cause mortality in the 12 weeks following COVID-19 vaccination compared to more than 12 weeks after any dose. However, we find an increase in cardiac death in women after a first dose of non mRNA vaccines. A positive SARS-CoV-2 test is associated with increased cardiac and all-cause mortality among people vaccinated or unvaccinated at time of testing.
© 2023. Crown.
Conflict of interest statement
K.K. is a member of the Ethnicity Subgroup of the UK Scientific Advisory Group for Emergencies (SAGE) and Member of SAGE. The remaining authors declare no competing interests.
Figures



References
-
- Department of Health and Social Care, Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. 2020. [Online].Available: https://www.gov.uk/government/publications/priority-groups-for-coronavir....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

