Altered behavior, brain structure, and neurometabolites in a rat model of autism-specific maternal autoantibody exposure
- PMID: 36973347
- PMCID: PMC10575787
- DOI: 10.1038/s41380-023-02020-3
Altered behavior, brain structure, and neurometabolites in a rat model of autism-specific maternal autoantibody exposure
Abstract
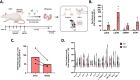
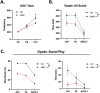
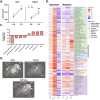
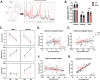
Maternal immune dysregulation is a prenatal risk factor for autism spectrum disorder (ASD). Importantly, a clinically relevant connection exists between inflammation and metabolic stress that can result in aberrant cytokine signaling and autoimmunity. In this study we examined the potential for maternal autoantibodies (aAbs) to disrupt metabolic signaling and induce neuroanatomical changes in the brains of exposed offspring. To accomplish this, we developed a model of maternal aAb exposure in rats based on the clinical phenomenon of maternal autoantibody-related ASD (MAR-ASD). Following confirmation of aAb production in rat dams and antigen-specific immunoglobulin G (IgG) transfer to offspring, we assessed offspring behavior and brain structure longitudinally. MAR-ASD rat offspring displayed a reduction in pup ultrasonic vocalizations and a pronounced deficit in social play behavior when allowed to freely interact with a novel partner. Additionally, longitudinal in vivo structural magnetic resonance imaging (sMRI) at postnatal day 30 (PND30) and PND70, conducted in a separate cohort of animals, revealed sex-specific differences in total and regional brain volume. Treatment-specific effects by region appeared to converge on midbrain and cerebellar structures in MAR-ASD offspring. Simultaneously, in vivo 1H magnetic resonance spectroscopy (1H-MRS) data were collected to examine brain metabolite levels in the medial prefrontal cortex. Results showed that MAR-ASD offspring displayed decreased levels of choline-containing compounds and glutathione, accompanied by increased taurine compared to control animals. Overall, we found that rats exposed to MAR-ASD aAbs present with alterations in behavior, brain structure, and neurometabolites; reminiscent of findings observed in clinical ASD.
© 2023. The Author(s).
Conflict of interest statement
JVW has a patent application involving the MAR-ASD peptides described herein and has a UC Davis-based startup company focusing on the development of the MAR-ASD autoantibody profile as a risk assessment for a child developing ASD. All other authors have no conflicts of interest to declare.
Figures

References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Arlington, VA; 2013.

