Discrimination between cyclic nucleotides in a cyclic nucleotide-gated ion channel
- PMID: 36973509
- PMCID: PMC10194703
- DOI: 10.1038/s41594-023-00955-3
Discrimination between cyclic nucleotides in a cyclic nucleotide-gated ion channel
Abstract
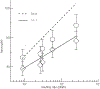
Cyclic nucleotide-gated ion channels are crucial in many physiological processes such as vision and pacemaking in the heart. SthK is a prokaryotic homolog with high sequence and structure similarities to hyperpolarization-activated and cyclic nucleotide-modulated and cyclic nucleotide-gated channels, especially at the level of the cyclic nucleotide binding domains (CNBDs). Functional measurements showed that cyclic adenosine monophosphate (cAMP) is a channel activator while cyclic guanosine monophosphate (cGMP) barely leads to pore opening. Here, using atomic force microscopy single-molecule force spectroscopy and force probe molecular dynamics simulations, we unravel quantitatively and at the atomic level how CNBDs discriminate between cyclic nucleotides. We find that cAMP binds to the SthK CNBD slightly stronger than cGMP and accesses a deep-bound state that a cGMP-bound CNBD cannot reach. We propose that the deep binding of cAMP is the discriminatory state that is essential for cAMP-dependent channel activation.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures


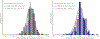
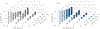


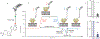

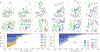
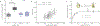

References
-
- Kaupp UB, Seifert R. Cyclic Nucleotide-Gated Ion Channels. Physiological Reviews 82, 769–824 (2002). - PubMed
-
- Craven KB, Zagotta WN. CNG AND HCN CHANNELS: Two Peas, One Pod. Annual Review of Physiology 68, 375–401 (2006). - PubMed
-
- Robinson RB, Siegelbaum SA. Hyperpolarization-Activated Cation Currents: From Molecules to Physiological Function. Annual Review of Physiology 65, 453–480 (2003). - PubMed
-
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-Activated Cation Channels: From Genes to Function. Physiological Reviews 89, 847–885 (2009). - PubMed
METHODS-ONLY REFERENCES
-
- Asakawa H, Yoshioka S, Nishimura K-i, Fukuma T. Spatial Distribution of Lipid Headgroups and Water Molecules at Membrane/Water Interfaces Visualized by Three-Dimensional Scanning Force Microscopy. ACS Nano 6, 9013–9020 (2012). - PubMed
-
- Miyagi A, Scheuring S. Automated force controller for amplitude modulation atomic force microscopy. Review of Scientific Instruments 87, 053705 (2016). - PubMed
-
- Miyagi A, Scheuring S. A novel phase-shift-based amplitude detector for a high-speed atomic force microscope. Review of Scientific Instruments 89, 083704 (2018). - PubMed
-
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234, 779–815 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

