Intrinsic activity development unfolds along a sensorimotor-association cortical axis in youth
- PMID: 36973514
- PMCID: PMC10406167
- DOI: 10.1038/s41593-023-01282-y
Intrinsic activity development unfolds along a sensorimotor-association cortical axis in youth
Abstract
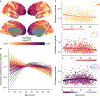
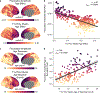
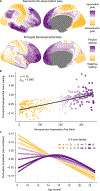
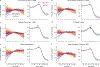
Animal studies of neurodevelopment have shown that recordings of intrinsic cortical activity evolve from synchronized and high amplitude to sparse and low amplitude as plasticity declines and the cortex matures. Leveraging resting-state functional MRI (fMRI) data from 1,033 youths (ages 8-23 years), we find that this stereotyped refinement of intrinsic activity occurs during human development and provides evidence for a cortical gradient of neurodevelopmental change. Declines in the amplitude of intrinsic fMRI activity were initiated heterochronously across regions and were coupled to the maturation of intracortical myelin, a developmental plasticity regulator. Spatiotemporal variability in regional developmental trajectories was organized along a hierarchical, sensorimotor-association cortical axis from ages 8 to 18. The sensorimotor-association axis furthermore captured variation in associations between youths' neighborhood environments and intrinsic fMRI activity; associations suggest that the effects of environmental disadvantage on the maturing brain diverge most across this axis during midadolescence. These results uncover a hierarchical neurodevelopmental axis and offer insight into the progression of cortical plasticity in humans.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare the following competing interest: RTS receives consulting income from Octave Bioscience for work wholly unrelated to the present research. All other authors declare no competing interests.
Figures


References
METHODS-ONLY REFERENCES
-
- Dale AM, Fischl B & Sereno MI Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9, 179–194 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
- RF1 MH121867/MH/NIMH NIH HHS/United States
- R37 MH125829/MH/NIMH NIH HHS/United States
- R01 MH123563/MH/NIMH NIH HHS/United States
- R01 MH120482/MH/NIMH NIH HHS/United States
- K08 MH120564/MH/NIMH NIH HHS/United States
- RF1 MH116920/MH/NIMH NIH HHS/United States
- RC2 MH089924/MH/NIMH NIH HHS/United States
- R01 MH113550/MH/NIMH NIH HHS/United States
- T32 MH014654/MH/NIMH NIH HHS/United States
- R01 MH120174/MH/NIMH NIH HHS/United States
- R01 MH119219/MH/NIMH NIH HHS/United States
- R01 MH119185/MH/NIMH NIH HHS/United States
- R01 EB022573/EB/NIBIB NIH HHS/United States
- R01 MH112847/MH/NIMH NIH HHS/United States
- R34 DA050297/DA/NIDA NIH HHS/United States
- K99 MH127293/MH/NIMH NIH HHS/United States
- R01 NS060910/NS/NINDS NIH HHS/United States
- RC2 MH089983/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

