Comprehensive analysis of scRNA-Seq and bulk RNA-Seq reveals dynamic changes in the tumor immune microenvironment of bladder cancer and establishes a prognostic model
- PMID: 36973787
- PMCID: PMC10044739
- DOI: 10.1186/s12967-023-04056-z
Comprehensive analysis of scRNA-Seq and bulk RNA-Seq reveals dynamic changes in the tumor immune microenvironment of bladder cancer and establishes a prognostic model
Abstract
Background: The prognostic management of bladder cancer (BLCA) remains a great challenge for clinicians. Recently, bulk RNA-seq sequencing data have been used as a prognostic marker for many cancers but do not accurately detect core cellular and molecular functions in tumor cells. In the current study, bulk RNA-seq and single-cell RNA sequencing (scRNA-seq) data were combined to construct a prognostic model of BLCA.
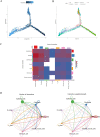
Methods: BLCA scRNA-seq data were downloaded from Gene Expression Omnibus (GEO) database. Bulk RNA-seq data were obtained from the UCSC Xena. The R package "Seurat" was used for scRNA-seq data processing, and the uniform manifold approximation and projection (UMAP) were utilized for downscaling and cluster identification. The FindAllMarkers function was used to identify marker genes for each cluster. The limma package was used to obtain differentially expressed genes (DEGs) affecting overall survival (OS) in BLCA patients. Weighted gene correlation network analysis (WGCNA) was used to identify BLCA key modules. The intersection of marker genes of core cells and genes of BLCA key modules and DEGs was used to construct a prognostic model by univariate Cox and Least Absolute Shrinkage and Selection Operator (LASSO) analyses. Differences in clinicopathological characteristics, immune microenvironment, immune checkpoints, and chemotherapeutic drug sensitivity between the high and low-risk groups were also investigated.
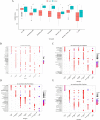
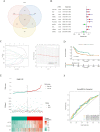
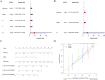
Results: scRNA-seq data were analyzed to identify 19 cell subpopulations and 7 core cell types. The ssGSEA showed that all 7 core cell types were significantly downregulated in tumor samples of BLCA. We identified 474 marker genes from the scRNA-seq dataset, 1556 DEGs from the Bulk RNA-seq dataset, and 2334 genes associated with a key module identified by WGCNA. After performing intersection, univariate Cox, and LASSO analysis, we obtained a prognostic model based on the expression levels of 3 signature genes, namely MAP1B, PCOLCE2, and ELN. The feasibility of the model was validated by an internal training set and two external validation sets. Moreover, patients with high-risk scores are predisposed to experience poor OS, a larger prevalence of stage III-IV, a greater TMB, a higher infiltration of immune cells, and a lesser likelihood of responding favorably to immunotherapy.
Conclusion: By integrating scRNA-seq and bulk RNA-seq data, we constructed a novel prognostic model to predict the survival of BLCA patients. The risk score is a promising independent prognostic factor that is closely correlated with the immune microenvironment and clinicopathological characteristics.
Keywords: Bladder cancer; Bulk RNA-seq; Immune landscape; Prognosis; scRNA-seq.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

