Inhibitory effect of Schisandrin on the pharmacokinetics of poziotinib in vivo and in vitro by UPLC-MS/MS
- PMID: 36973912
- PMCID: PMC10175034
- DOI: 10.1111/1759-7714.14870
Inhibitory effect of Schisandrin on the pharmacokinetics of poziotinib in vivo and in vitro by UPLC-MS/MS
Abstract
Background: As a pan-HER tyrosine kinase inhibitor with a promising application prospect, poziotinib is likely to be coadministered with Schisandrins in clinical treatment due to its anticancer activities.
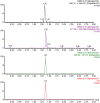
Methods: Eighteen Sprague-Dawley rats were randomly divided into three groups: Schisandrin A group and Schisandrin B group (20 mg/kg daily for 1 week), and control group (vehicle). On day 8, poziotinib (2 mg/kg) was administered by oral gavage 30 min later. An in vitro study was developed to identify the possible mechanisms of Schisandrins on poziotinib metabolism. All analytes were detected by UPLC/MS-MS, and molecular docking was performed by AutoDock Tools.
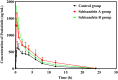
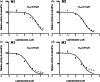
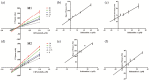
Results: When rats were preadministered with Schisandrin A, AUC(0-∞) and Cmax of poziotinib were obviously increased by 0.79- and 1.17-fold, whereas the Vz/F and CLz/F values were dramatically decreased. The results in Schisandrin B group presented similarly. Both Schisandrin A and Schisandrin B were mixed inhibitors of poziotinib in RLMs, and Schisandrin B showed stronger inhibitory activity with IC50 values of 2.55 μM for M1 and 6.97 μM for M2. Molecular docking analysis demonstrated that Schisandrin A and Schisandrin B exhibited a strong binding ability towards CYP2D6 as compared to CYP3A4.
Conclusion: All results provided the direct evidence of the pharmacokinetic drug-drug interactions (DDIs) between Schisandrin and poziotinib. Thus, particular attention should be paid when poziotinib is taken together with Schisandrins in clinical practice.
Keywords: drug-drug interactions; molecular docking; pharmacokinetic; poziotinib; schisandrin.
© 2023 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors have no conflicts of interest and no disclosures to declare.
Figures



Similar articles
-
Effects of dacomitinib on the pharmacokinetics of poziotinib in vivo and in vitro.Pharm Biol. 2021 Dec;59(1):457-464. doi: 10.1080/13880209.2021.1914114. Pharm Biol. 2021. PMID: 33899675 Free PMC article.
-
Assessments of CYP‑inhibition‑based drug-drug interaction between vonoprazan and poziotinib in vitro and in vivo.Pharm Biol. 2023 Dec;61(1):356-361. doi: 10.1080/13880209.2023.2173253. Pharm Biol. 2023. PMID: 36728978 Free PMC article.
-
Effects of Avitinib on CYP450 Enzyme Activity in vitro and in vivo in Rats.Drug Des Devel Ther. 2021 Aug 21;15:3661-3673. doi: 10.2147/DDDT.S323186. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34456561 Free PMC article.
-
Investigation of pharmacokinetics, tissue distribution and excretion of schisandrin B in rats by HPLC-MS/MS.Biomed Chromatogr. 2018 Feb;32(2). doi: 10.1002/bmc.4069. Epub 2017 Sep 24. Biomed Chromatogr. 2018. PMID: 28833320
-
Effect of apatinib on the pharmacokinetics of tramadol and O-desmethyltramadol in rats.PeerJ. 2023 Sep 11;11:e16051. doi: 10.7717/peerj.16051. eCollection 2023. PeerJ. 2023. PMID: 37719112 Free PMC article.
Cited by
-
Drug-drug interactions between epidermal growth factor receptor tyrosine kinase inhibitors and rivaroxaban in vitro and in vivo.PLoS One. 2025 Jun 3;20(6):e0322303. doi: 10.1371/journal.pone.0322303. eCollection 2025. PLoS One. 2025. PMID: 40460109 Free PMC article.
References
-
- Kim TY, Han HS, Lee KW, Zang DY, Rha SY, Park YI, et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2‐positive advanced gastric cancer. Gastric Cancer. 2019;22:1206–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

