Scalable Synthesis of Planar Macroscopic Lipid-Based Multi-Compartment Structures
- PMID: 36973945
- PMCID: PMC10100540
- DOI: 10.1021/acs.langmuir.2c02859
Scalable Synthesis of Planar Macroscopic Lipid-Based Multi-Compartment Structures
Abstract
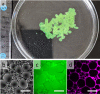
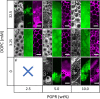
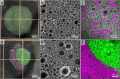
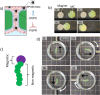
As life evolved, the path from simple single cell organisms to multicellular enabled increasingly complex functionalities. The spatial separation of reactions at the micron scale achieved by cellular structures allowed diverse and scalable implementation in biomolecular systems. Mimicking such spatially separated domains in a scalable approach could open a route to creating synthetic cell-like structured systems. Here, we report a facile and scalable method to create multicellular-like, multi-compartment (MC) structures. Aqueous droplet-based compartments ranging from 50 to 400 μm were stabilized and connected together by hydrophobic layers composed of phospholipids and an emulsifier. Planar centimeter-scale MC structures were formed by droplet deposition on a water interface. Further, the resulting macroscopic shapes were shown to be achieved by spatially controlled deposition. To demonstrate configurability and potential versatility, MC assemblies of both homogeneous and mixed compartment types were shown. Notably, magnetically heterogeneous systems were achieved by the inclusion of magnetic nanoparticles in defined sections. Such structures demonstrated actuated motion with structurally imparted directionality. These novel and functionalized structures exemplify a route toward future applications including compartmentally assembled "multicellular" molecular robots.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

