Pilot Biomarker Analysis and Decision Tree Algorithm Modeling of Patients with Chronic Subdural Hematomas
- PMID: 36974123
- PMCID: PMC10039273
- DOI: 10.1089/neur.2022.0062
Pilot Biomarker Analysis and Decision Tree Algorithm Modeling of Patients with Chronic Subdural Hematomas
Abstract
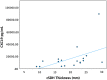
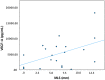
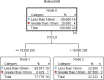
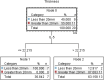
The elderly population are at high risk for developing chronic subdural hematoma (cSDH). Surgical evacuation of cSDH is one of the most common procedures performed in neurosurgery. The present study aims to identify potential inflammatory biomarkers associated with its development and recurrence. Patients (>65 years of age) who presented with symptomatic cSDH (≥1 cm thickness or ≥5 mm midline shift [MLS]), requiring surgical intervention, were prospectively enrolled. The collected cSDH fluid was analyzed for inflammatory markers. Computed tomography (CT) scan data included pre-operative cSDH thickness and MLS. Outcome data included Glasgow Outcome Scale-Extended (GOS-E) score at 3, 6, and 12 months post-surgery, as well as cSDH recurrence. A decision tree model was used to determine the predictive power of extracted analytes for MLS, cSDH thickness, and recurrence. This pilot study includes 20 enrolled patients (mean age 77.9 ± 7.4 years and 85% falls). Rate of cSDH recurrence was 42%, with 21% requiring reoperation. Chemokine (C-X-C motif) ligand 9 (CXCL9) concentrations correlated with cSDH thickness (r = 0.975, p = 0.040). Interleukin (IL)-6 and vascular endothelial growth factor (VEGF)-A concentrations correlated with MLS (r = 0.974, p = 0.005; r = 0.472, p = 0.036, respectively). IL-5 concentrations correlated with more favorable GOS-E scores at 3, 6, and 12 months (r = 0.639, p = 0.006; r = 0.727, p = 0.003; r = 0.693, p = 0.026, respectively). Regulated on activation, normal T-cell expressed and secreted (RANTES) concentrations correlated with complete cSDH resolution (r = 0.514, p = 0.021). The decision tree model identified that higher concentrations of CXCL9 were predictive of MLS (risk ratio [RR] = 12.0), higher concentrations of IL-5 were predictive of cSDH thickness (RR = 4.5), and lower concentrations of RANTES were predictive of cSDH recurrence (RR = 2.2). CXCL9, IL-6, VEGF, IL-5, and RANTES are associated with recurrence after surgery and may be potential biomarkers for predicting cSDH recurrence and neurological outcomes.
Keywords: CT imaging; Glasgow Outcome Scale-Extended; biomarker; chronic subdural hematoma; inflammation; traumatic brain injury.
© David J. Puccio et al., 2023; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
Biofluid Biomarkers in the Prognosis of Chronic Subdural Hematoma: A Systematic Scoping Review.Diagnostics (Basel). 2023 Jul 22;13(14):2449. doi: 10.3390/diagnostics13142449. Diagnostics (Basel). 2023. PMID: 37510193 Free PMC article.
-
Comparative Study of Single Burr-Hole Craniostomy versus Twist-Drill Craniostomy in Patients with Chronic Subdural Hematoma.Asian J Neurosurg. 2019 Apr-Jun;14(2):513-521. doi: 10.4103/ajns.AJNS_37_19. Asian J Neurosurg. 2019. PMID: 31143272 Free PMC article.
-
Transcranial color-coded duplex sonography for evaluation of midline-shift after chronic-subdural hematoma evacuation (TEMASE): A prospective study.Clin Neurol Neurosurg. 2017 Nov;162:101-107. doi: 10.1016/j.clineuro.2017.09.015. Epub 2017 Oct 3. Clin Neurol Neurosurg. 2017. PMID: 29017105
-
The Mini-Craniotomy for cSDH Revisited: New Perspectives.Front Neurol. 2021 May 6;12:660885. doi: 10.3389/fneur.2021.660885. eCollection 2021. Front Neurol. 2021. PMID: 34025564 Free PMC article.
-
A Radiological Assessment of Chronic Subdural Hematomas.Korean J Neurotrauma. 2022 Apr 25;18(1):12-21. doi: 10.13004/kjnt.2022.18.e24. eCollection 2022 Apr. Korean J Neurotrauma. 2022. PMID: 35557646 Free PMC article. Review.
Cited by
-
Biofluid Biomarkers in the Prognosis of Chronic Subdural Hematoma: A Systematic Scoping Review.Diagnostics (Basel). 2023 Jul 22;13(14):2449. doi: 10.3390/diagnostics13142449. Diagnostics (Basel). 2023. PMID: 37510193 Free PMC article.
References
-
- Karibe H, Kameyama M, Kawase M, et al. . [Epidemiology of chronic subdural hematomas]. [Article in Japanese]. No Shinkei Geka 2011;39(12):1149–1153. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
