Raptors bred in captivity: semen characteristics and assisted reproduction outcome in goshawk (Accipiter gentilis)
- PMID: 36974138
- PMCID: PMC10039655
- DOI: 10.7717/peerj.15094
Raptors bred in captivity: semen characteristics and assisted reproduction outcome in goshawk (Accipiter gentilis)
Abstract
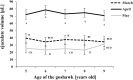
Three sexually mature goshawks reared in captivity and imprinted on humans to express reproductive behavior according to the cooperative method were studied for three consecutive breeding seasons to assess the quality of their sperm. The following parameters were analyzed: ejaculate volume and sperm concentration, motility, viability, and morphology. Ejaculate volume, sperm concentration and motility fluctuated along the reproductive season, revealing the greatest quality of the reproductive material at full springtime (i.e., April). Motility of the sperm collected in March strongly reduced with age, contrary to samples collected in April or May. Sperm viability was not influenced by either age or month of collection within each season. Ultrastructural investigations provided information on normal sperm morphology for the first time in this species. The morphological categories of sperm defects in fresh semen, present at low percentages, are also described. Functional analyses (perivitelline membrane assay and artificial inseminations) confirmed the good quality of the semen obtained using the cooperative method. The reported data provide the basis for further studies aimed at developing protocols to improve the outcome of artificial insemination and semen cryopreservation in the goshawk as well as other bird of prey species.
Keywords: Captive breeding; Goshawk; Raptor species conservation; Season; Semen characteristics; Sperm ultrastructure.
© 2023 Fausto et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Al-Daraji HJ, Al-Shemmary SA. Effect of breed falcon on semen quality traits. International Journal of Conservation Science. 2016;7:725–734.
-
- Alkan S, Baran A, Özdaş ÖB, Evecen M. Morphological defects in Turkey semen. Turkish Journal of Veterinary and Animal Sciences. 2002;26:1087–1092.
-
- Bakst M. Sperm viability. In: Bakst M, Long J, editors. Techniques for Semen Evaluation, Semen Storage, and Fertility Determination. Buffalo, Minnesota: The Midwest Poultry Federation; 2010. pp. 39–41.
-
- Bergonzoli S, Brambilla M, Romano E, Saia S, Cetera P, Cutini M, Toscano P, Bisaglia C, Pari L. Feeding emitters for microirrigation with a digestate liquid fraction up to 25% dilution did not reduce their performance. Agronomy. 2020;10(8):1–14. doi: 10.3390/agronomy10081150. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

