Health-related quality of life in cardiac sarcoidosis: a systematic review
- PMID: 36974155
- PMCID: PMC10039618
- DOI: 10.1093/ehjopen/oead009
Health-related quality of life in cardiac sarcoidosis: a systematic review
Abstract
People living with cardiac sarcoidosis (CS) are likely to have worse clinical outcomes and greater impairment on health-related quality of life (HRQoL) than other sarcoidosis manifestations. CS can result in a constellation of intrusive symptoms (such as palpitations, dizziness, syncope/pre-syncope, chest pain, dyspnoea, orthopnoea, or peripheral oedema) and/or life-threatening episodes, requiring consideration of invasive cardiac procedures for diagnosis and for the management of acute events. Additionally, the presence of multisystemic involvement and persistent non-specific sarcoidosis symptoms negatively affect HRQoL. A systematic review was undertaken to explore the impact of CS on HRQoL in adults with CS. Multiple bibliographic databases were searched for studies with HRQoL as primary or secondary outcomes in CS (PROSPERO registration: CRD42019119752). Data extraction and quality assessments were undertaken independently by two authors. From the initial 1609 identified records, only 11 studies included CS patients but none specifically reported HRQoL scores for CS patients. The average representation of CS patients was 14.5% within these cohorts (range 2-22%). The majority (73%) was conducted in single-centre tertiary care settings, and only one study (9%) included longitudinal HRQoL data. CS patients were among those sarcoidosis patients with impaired HRQoL and worse outcomes, requiring higher doses of sarcoidosis-specific therapy which contribute to further deterioration of HRQoL. Sarcoidosis studies do not incorporate stratified HRQoL scores for CS patients. While there is a need for longitudinal and multicentre studies assessing HRQoL outcomes in CS cohorts, the development of CS-specific tools is also needed.
Keywords: Cardiac sarcoidosis; Health-related quality of life (HRQoL); Patient-reported outcome measures (PROMS); Systematic review.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: J.S. is the primary supervisor for the award NIHR301572 and is supported to attend European Society of Cardiology (ESC) meetings as board member for the Association for Cardiovascular Nursing and Allied Professions (ACNAP). The other authors declare no conflict of interest relevant to this article.
Figures



Limited CS representation (4–5%), socio-demographically imbalanced characteristics, scores for the SF-36 subscales not provided.,
Limited CS representation (8%), no details were provided for the 25% of the sarcoidosis population who did not return study questionnaires.
Limited CS representation (3%), two references were used to describe the cohort of participants, but the provided samples were not matching with the reported data.
Limited CS representation (2%).
Online survey with no clinical characteristics. Self-reported socio-demographic characteristics. Only the SHQ total score was provided.
Retrospective analysis. Over half (363/660, 55%) of the cohort did not complete the SAT due to the validation date.
Monthly self-completed assessments using a new online platform for 6 months, and the study only reported baseline HRQoL scores.
Limited CS representation (7%). Only patients undergoing pulmonary function test during the clinical routine visits were eligible. No confidence intervals included.
Data analysed retrospectively from a multi-national database (n = 456). Participants with advanced sarcoidosis (n = 231) were followed up every 6 months for 3 years, but only baseline HRQoL assessments were reported. The analysis included over one-half (53%) of the cohort with advanced sarcoidosis and completed a 12-month visit.
Participants completed a follow-up 5-year visit, but HRQoL assessments were included only at baseline.
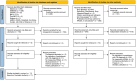
Other bias was deemed as unclear risk for all the studies considering that none of the studies fulfilled the full CASP checklist for cohort studies as detailed in Figure 2.
References
-
- Hamzeh N, Steckman DA, Sauer WH, Judson MA. Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol 2015;12:278–288. - PubMed
-
- Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. Am J Med 1977;63:86–108. - PubMed
-
- Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, PietiläEffati P, Utrianen S, Kupari M. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624–632. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

