Microbial community succession and volatile compounds changes during spontaneous fermentation of Cabernet Sauvignon (Vitis vinifera L.) under rain-shelter cultivation
- PMID: 36974178
- PMCID: PMC10039258
- DOI: 10.1016/j.fochx.2023.100618
Microbial community succession and volatile compounds changes during spontaneous fermentation of Cabernet Sauvignon (Vitis vinifera L.) under rain-shelter cultivation
Abstract
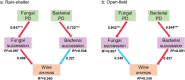
Microbiota succession in spontaneous fermentation of Cabernet Sauvignon cultivated under the rain-shelter was characterized, with open-field cultivation as the control. For both cultivation modes, Saccharomyces, Starmerella, and Mycosphearella were the principal fungi, and Tatumella, Gluconobacter, and Acinetobacter were the prevailing bacteria. Rain-shelter reduced the abundance of Hanseniaspora, Candida, Starmerella, Gluconobacter, and Lactococcus. During fermentation, fungal microbiota diversity in samples from the rain-shelter cultivation decreased more drastically than the control (p < 0.05). In terms of the correlation between microbiota and volatile compounds production, the abundance of Hanseniaspora uvarum, Candida apicola, Starmerella bacillaris, Gluconobacter oxydans, and Lactococcus lactis were positively correlated with the production of esters and higher alcohols. Instead of bacterial microbiota, fungal community succession exhibited a positive correlation with the final wine volatiles under the rain-shelter cultivation. These findings demonstrated rain-shelter cultivation influences the succession pattern of microbial communities and in turn impacts the wine aromas and flavors.
Keywords: Correlation analysis; Flavor; Microbial diversity; Spontaneous fermentation; Volatile compounds.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Andorrà I., Berradre M., Mas A., Esteve-Zarzoso B., Guillamón J.M. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT. 2012;49(1):8–13. doi: 10.1016/j.lwt.2012.04.008. - DOI
-
- Bian X., Chen J.-R., Fan J., Yang Y., Yu D.-H., Ren L.-K.…Zhang N. Microbial and genes diversity analysis: Relationship between starch conversion and carbohydrate metabolism during Niandoubao fermentation via the glutinous proso millet (GPM) process. Food Control. 2022;140 doi: 10.1016/j.foodcont.2022.109154. - DOI
LinkOut - more resources
Full Text Sources

