The Clinical Efficacy, Safety, and Tolerability of Vancomycin for the Treatment of Recurrent Clostridioides difficile Infection - A Systematic Review
- PMID: 36974197
- PMCID: PMC10039659
- DOI: 10.2147/DHPS.S348501
The Clinical Efficacy, Safety, and Tolerability of Vancomycin for the Treatment of Recurrent Clostridioides difficile Infection - A Systematic Review
Abstract
Introduction: The aim of this systematic review of randomized clinical trials (RCTs) was to examine the efficacy, safety, and tolerability of vancomycin for treatment of recurrent Clostridioides difficile infection (rCDI).
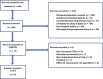
Methods: The PubMed database was searched from inception to August 23, 2022. An initial screening was performed followed by a full-text evaluation of the papers. Inclusion criteria were RCTs investigating vancomycin for treatment of rCDI.
Results: A total of six studies and 269 patients were included in the review. Three studies used a fixed dose regimen of vancomycin, one study used pulse regimen, one study used a taper-and-pulse regimen, and one study used a taper-and-pulse regimen for the participants with two or more recurrences. The resolution of infection varied from 19% to 58.3% in five of six studies reporting this as an outcome. Four out of six studies reported new episodes of rCDI as an intervention outcome, in those studies 50-63% of participants experienced rCDI. Regarding the safety and tolerability of vancomycin treatment for rCDI, one study described several adverse events regarding gastrointestinal discomfort along with fatigue and skin rash. There were no records of serious adverse events in the included studies.
Conclusion: While oral vancomycin is mostly safe and well tolerated in the RCTs reviewed here, the efficacy for treating rCDI varies greatly from 19-58.3%, and 50-63% of participants experienced new episodes of rCDI.
Keywords: Clostridioides difficile; diarrhoea; infection; recurrent; vancomycin.
© 2023 Knudsen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Prevention of recurrent Clostridioides difficile infection: A systematic review of randomized controlled trials.Anaerobe. 2020 Feb;61:102098. doi: 10.1016/j.anaerobe.2019.102098. Epub 2019 Sep 4. Anaerobe. 2020. PMID: 31493500
-
Defining optimal treatment for recurrent Clostridioides difficile infection (OpTION study): A randomized, double-blind comparison of three antibiotic regimens for patients with a first or second recurrence.Contemp Clin Trials. 2022 May;116:106756. doi: 10.1016/j.cct.2022.106756. Epub 2022 Apr 7. Contemp Clin Trials. 2022. PMID: 35398532 Clinical Trial.
-
Systematic Review and Meta-Analysis: Efficacy of Vancomycin Taper and Pulse Regimens in Clostridioides difficile Infection.Expert Rev Anti Infect Ther. 2022 Apr;20(4):577-583. doi: 10.1080/14787210.2022.1997588. Epub 2021 Nov 1. Expert Rev Anti Infect Ther. 2022. PMID: 34693838
-
Characterization and risk factors for recurrence of Clostridioides (Clostridium) difficile infection in Japan: A nationwide real-world analysis using a large hospital-based administrative dataset.J Infect Chemother. 2019 Aug;25(8):615-620. doi: 10.1016/j.jiac.2019.03.011. Epub 2019 Apr 12. J Infect Chemother. 2019. PMID: 30987950
-
Extended duration vancomycin in recurrent Clostridium difficile infection: a systematic review.Ther Adv Infect Dis. 2018 Sep 12;5(6):111-119. doi: 10.1177/2049936118798276. eCollection 2018 Nov. Ther Adv Infect Dis. 2018. PMID: 30430009 Free PMC article. Review.
Cited by
-
Therapeutics involved in managing initial and recurrent Clostridium difficile infection: An updated literature review.World J Gastrointest Pharmacol Ther. 2024 Sep 5;15(5):95467. doi: 10.4292/wjgpt.v15.i5.95467. World J Gastrointest Pharmacol Ther. 2024. PMID: 39281262 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

