Inhaled Corticosteroids in Patients with Chronic Obstructive Pulmonary Disease and Risk of Acquiring Streptococcus pneumoniae Infection. A Multiregional Epidemiological Study
- PMID: 36974273
- PMCID: PMC10039661
- DOI: 10.2147/COPD.S386518
Inhaled Corticosteroids in Patients with Chronic Obstructive Pulmonary Disease and Risk of Acquiring Streptococcus pneumoniae Infection. A Multiregional Epidemiological Study
Abstract
Background: Inhaled corticosteroids (ICS) are associated with an increased risk of clinical pneumonia among patients with chronic obstructive pulmonary disease (COPD). It is unknown whether the risk of microbiologically verified pneumonia such as pneumococcal pneumonia is increased in ICS users.
Methods: The study population consists of all COPD patients followed in outpatient clinics in eastern Denmark during 2010-2017. ICS use was categorized into four categories based on accumulated use. A Cox proportional hazard regression model was used adjusting for age, body mass index, sex, airflow limitation, use of oral corticosteroids, smoking, and year of cohort entry. A propensity score matched analysis was performed for sensitivity analyses.
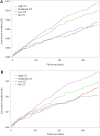
Findings: A total of 21,438 patients were included. Five hundred and eighty-two (2.6%) patients acquired a positive lower airway tract sample with S. pneumoniae during follow-up. In the multivariable analysis ICS-use was associated with a dose-dependent risk of S. pneumoniae as follows: low ICS dose: HR 1.11, 95% CI 0.84 to 1.45, p = 0.5; moderate ICS dose: HR 1.47, 95% CI 1.13 to 1.90, p = 0.004; high ICS dose: HR 1.77, 95% CI 1.38 to 2.29, p < 0.0001, compared to no ICS use. Sensitivity analyses confirmed these results.
Interpretation: Use of ICS in patients with severe COPD was associated with an increased and dose-dependent risk of acquiring S. pneumoniae, but only for moderate and high dose. Caution should be taken when administering high dose of ICS to patients with COPD. Low dose of ICS seemed not to carry this risk.
Keywords: COPD; Streptococcus pneumoniae; clinical epidemiology; inhaled corticosteroids.
© 2023 Heerfordt et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding this article. However, to ensure full transparency, we disclose the following potential conflicts of interest: PS received payment for lectures by Boehringer Ingelheim AstraZeneca, and GSK. TBS received grants from Sanofi Pasteur, Novo Nordisk Foundation, and Lundbeck foundation, received Consulting fees from Sanofi Pasteur, and Amgen, and received payment for lectures by Sanofi Pasteur, and GSK. TBS participates on Data Safety Monitoring Board or Advisory Board for Sanofi Pasteur and Amgen. ZBH received support for meetings and/or travel by Phizer. AKB received support for meetings and/or travel by PulmonX and reports non-financial support from GSK, during the conduct of the study. AGM received grants from Boehringer Ingelheim and is associated with the startup “Healthy network” producing automated e-stethoscopes. JRH received consulting fees from AstraZeneca, received payment for lectures by Boehringer Ingelheim, and Takeda, and received support to meetings and/or travel by AstraZeneca, and participates in AstraZeneca’s Advisory board and British Thoracic Society. MP received grants from Roche, received consulting fees from Takeda, Therakos, Zambon, and received payment for lectures by Mallinckrodt, PulmonX, and Glaxo-smith Kline, received support for meetings and/or travel by Boehringer-Ingelheim and is Chair of Danish transplant Society, and associated with The ERS group for transplantation. All other authors declare no competing interests in this work.
Figures

References
-
- Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi:10.1016/S0140-6736(16)30069-1 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

