Herbal supplements as treatment options for COVID-19: A call for clinical development of herbal supplements for emerging and re-emerging viral threats in Sub-Saharan Africa
- PMID: 36974333
- PMCID: PMC9985929
- DOI: 10.1016/j.sciaf.2023.e01627
Herbal supplements as treatment options for COVID-19: A call for clinical development of herbal supplements for emerging and re-emerging viral threats in Sub-Saharan Africa
Abstract
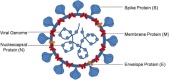
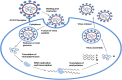
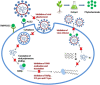
The advent of Corona virus Disease 2019 (COVID-19) distorted health systems of many countries. Efforts have been made to either develop new treatment solutions such as vaccines or repurpose previously adopted drugs. Challenges in accessing available treatment, inadequate, non-existent, or overstretched healthcare facilities, long COVID disease, cultural practices and beliefs about vaccination, vaccine hesitancy, availability, accessibility and perceived safety of herbal supplements seem to be major factors propelling individuals to use herbal supplements. Published reports advocating for clinical development of herbal supplements for COVID-19 and other emerging and re-emerging viral diseases are sparse. This paper aims to review the pathogenesis of COVID-19, use of herbal products during the pandemic and make case for clinical development of herbal supplements through the adoption of modern and acceptable technologies and research processes. This was a scoping review. Database searches of Google Scholar, PubMed and ResearchGate among others were performed using related keywords to identify relevant journals and lists of primary articles. Clinical trial databases:-Clinicaltrial.gov, Pan African Clinical Trial Registry (PACTR) and WHO international clinical trial registry (ICTRP) were reviewed to extract data. The use of herbal supplements during COVID-19 was not only peculiar to individuals living in Sub-Saharan Africa, but a global practice. Herbal supplements recommended to manage COVID-19 have not been validated using clinical trials. Available data showed that the number of herbal supplements undergoing clinical trial for COVID-19 indication in Africa was low. The availability of medicinal plants in Sub-Saharan Africa if well explored has great potentials to address various emerging and re-emerging viral diseases confronting the region. The economic potential of clinically validated herbal supplements are huge, and tapping into this opportunity created by preference of population to herbal supplement could increase export of herbal supplement and gross domestic product (GDP) of respective countries in Africa.
Keywords: COVID-19; Clinical Development; Herbal Medicine; Herbal Products; Herbal Supplements; SARS-CoV-2.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- El-Sadr W.M., Justman J. Africa in the path of Covid-19. N. Engl. J. Med. 2020;383(3):e11. - PubMed
-
- World Health Organization, Declaration of alma-ata. (1978). Available at: https://www.who.int/teams/social-determinants-of-health/declaration-of-a.... Retrieved September 30, 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous
