Cortical iron accumulation in MAPT- and C9orf 72-associated frontotemporal lobar degeneration
- PMID: 36974379
- PMCID: PMC10307524
- DOI: 10.1111/bpa.13158
Cortical iron accumulation in MAPT- and C9orf 72-associated frontotemporal lobar degeneration
Abstract
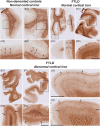
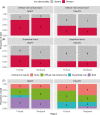
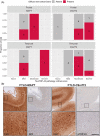
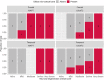
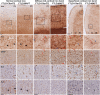
Neuroinflammation has been implicated in frontotemporal lobar degeneration (FTLD) pathophysiology, including in genetic forms with microtubule-associated protein tau (MAPT) mutations (FTLD-MAPT) or chromosome 9 open reading frame 72 (C9orf72) repeat expansions (FTLD-C9orf72). Iron accumulation as a marker of neuroinflammation has, however, been understudied in genetic FTLD to date. To investigate the occurrence of cortical iron accumulation in FTLD-MAPT and FTLD-C9orf72, iron histopathology was performed on the frontal and temporal cortex of 22 cases (11 FTLD-MAPT and 11 FTLD-C9orf72). We studied patterns of cortical iron accumulation and its colocalization with the corresponding underlying pathologies (tau and TDP-43), brain cells (microglia and astrocytes), and myelination. Further, with ultrahigh field ex vivo MRI on a subset (four FTLD-MAPT and two FTLD-C9orf72), we examined the sensitivity of T2*-weighted MRI for iron in FTLD. Histopathology showed that cortical iron accumulation occurs in both FTLD-MAPT and FTLD-C9orf72 in frontal and temporal cortices, characterized by a diffuse mid-cortical iron-rich band, and by a superficial cortical iron band in some cases. Cortical iron accumulation was associated with the severity of proteinopathy (tau or TDP-43) and neuronal degeneration, in part with clinical severity, and with the presence of activated microglia, reactive astrocytes and myelin loss. Ultra-high field T2*-weighted MRI showed a good correspondence between hypointense changes on MRI and cortical iron observed on histology. We conclude that iron accumulation is a feature of both FTLD-MAPT and FTLD-C9orf72 and is associated with pathological severity. Therefore, in vivo iron imaging using T2*-weighted MRI or quantitative susceptibility mapping may potentially be used as a noninvasive imaging marker to localize pathology in FTLD.
Keywords: C9orf72; MAPT; frontotemporal lobar degeneration; iron accumulation; neuroinflammation.
© 2023 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Figures

References
-
- Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82:476–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

