Non-Arteritic Ischemic Optic Neuropathy Following COVID-19 Vaccination in Korea: A Case Series
- PMID: 36974402
- PMCID: PMC10042731
- DOI: 10.3346/jkms.2023.38.e95
Non-Arteritic Ischemic Optic Neuropathy Following COVID-19 Vaccination in Korea: A Case Series
Abstract
Background: To report the clinical manifestations of non-arteritic anterior ischemic optic neuropathy (NAION) cases after coronavirus disease 2019 (COVID-19) vaccination in Korea.
Methods: This multicenter retrospective study included patients diagnosed with NAION within 42 days of COVID-19 vaccination. We collected data on vaccinations, demographic features, presence of vascular risk factors, ocular findings, and visual outcomes of patients with NAION.
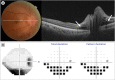
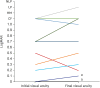
Results: The study included 16 eyes of 14 patients (6 men, 8 women) with a mean age of 63.5 ± 9.1 (range, 43-77) years. The most common underlying disease was hypertension, accounting for 28.6% of patients with NAION. Seven patients (50.0%) had no vascular risk factors for NAION. The mean time from vaccination to onset was 13.8 ± 14.2 (range, 1-41) days. All 16 eyes had disc swelling at initial presentation, and 3 of them (18.8%) had peripapillary intraretinal and/or subretinal fluid with severe disc swelling. Peripapillary hemorrhage was found in 50% of the patients, and one (6.3%) patient had peripapillary cotton-wool spots. In eight fellow eyes for which we were able to review the fundus photographs, the horizontal cup/disc ratio was less than 0.25 in four eyes (50.0%). The mean visual acuity was logMAR 0.6 ± 0.7 at the initial presentation and logMAR 0.7 ± 0.8 at the final visit.
Conclusion: Only 64% of patients with NAION after COVID-19 vaccination have known vascular and ocular risk factors relevant to ischemic optic neuropathy. This suggests that COVID-19 vaccination may increase the risk of NAION. However, overall clinical features and visual outcomes of the NAION patients after COVID-19 vaccination were similar to those of typical NAION.
Keywords: Coronavirus Disease 2019 (COVID-19); Non-Arteritic Anterior Ischemic Optic Neuropathy; Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2); Vaccination.
© 2023 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

