Angina After Percutaneous Coronary Intervention: Patient and Procedural Predictors
- PMID: 36974680
- PMCID: PMC10101135
- DOI: 10.1161/CIRCINTERVENTIONS.122.012511
Angina After Percutaneous Coronary Intervention: Patient and Procedural Predictors
Abstract
Background: Twenty percent to 40% of patients are affected by angina after percutaneous coronary intervention (PCI), which is associated with anxiety, depression, impaired physical function, and reduced quality of life. Understanding patient and procedural factors associated with post-PCI angina may inform alternative approaches to treatment.
Methods: Two hundred thirty patients undergoing PCI completed the Seattle Angina Questionnaire (SAQ-7) and European quality of life-5 dimension-5 level (EQ-5D-5L) questionnaires at baseline and 3 months post-PCI. Patients received blinded intracoronary physiology assessments before and after stenting. A post hoc analysis was performed to compare clinical and procedural characteristics among patients with and without post-PCI angina (defined by follow-up SAQ-angina frequency score <100).
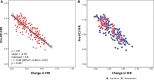
Results: Eighty-eight of 230 patients (38.3%) reported angina 3 months post-PCI and had a higher incidence of active smoking, atrial fibrillation, and history of previous myocardial infarction or PCI. Compared with patients with no angina at follow-up, they had lower baseline SAQ summary scores (69.48±24.12 versus 50.20±22.59, P<0.001) and EQ-5D-5L health index scores (0.84±0.15 versus 0.69±0.22, P<0.001). Pre-PCI fractional flow reserve (FFR) was lower among patients who had no post-PCI angina (0.56±0.15 versus 0.62±0.13, P=0.003). Percentage change in FFR after PCI had a moderate correlation with angina frequency score at follow-up (r=0.36, P<0.0001). Patients with post-PCI angina had less improvement in FFR (43.1±33.5% versus 67.0±50.7%, P<0.001). There were no between-group differences in post-PCI FFR, coronary flow reserve, or corrected index of microcirculatory resistance. Patients with post-PCI angina had lower SAQ-summary scores (64.01±22 versus 95.16±8.72, P≤0.001) and EQ-5D-5L index scores (0.69±0.26 versus 0.91±0.17, P≤0.001) at follow-up.
Conclusions: Larger improvements in FFR following PCI were associated with less angina and better quality of life at follow-up. In patients with stable symptoms, intracoronary physiology assessment can inform expectations of angina relief and quality of life improvement after stenting and thereby help to determine the appropriateness of PCI.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT03259815.
Keywords: angina pectoris; coronary artery disease; myocardial infarction; myocardium; percutaneous coronary intervention; quality of life.
Conflict of interest statement
Dr Collison received consultancy fees from Abbott. Dr Mizukami received consultancy fees from Zeon Medical Inc; research grants from Boston Scientific; speaker fees from Abbott, Cathworks, Boston Scientific. Dr Collet received research grants from Biosensors, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, Abbott; consultancies HeartFlow, OpSens, Abbott, Philips Volcano. Dr Ford received consulting fees from BioExcel; honoraria from Abbott, Boehringer, Novartis. Dr Watkins received honoraria from Abbott, AstraZeneca, Biosensors, Boston Scientific, Daiichi Sankyo, GE Healthcare, ShockWave Medical. Dr Robertson received honoraria from AstraZeneca, Abbott. Dr O’Boyle received honoraria from AstraZeneca, Boston Scientific, Novartis. Dr McEntegart received consulting fees from Abbott, Boston Scientific, ShockWave Medical; honoraria from International Medical Device Solutions, Medtronic. Prof Berry received institutional grants/contracts from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HeartFlow, Novartis, Siemens Healthcare; consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HeartFlow, Menarini, Novartis; honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HeartFlow, Philips, Valo Health. Prof Oldroyd received honoraria from Abbott, Biosensors International, Boston Scientific; institutional research grant from Boston Scientific which supported the present manuscript; full-time employee of Biosensors International since May 2020. The other authors report no conflicts.
Figures
Comment in
-
Can We Predict Who Will Have Angina Relief From Percutaneous Coronary Intervention?Circ Cardiovasc Interv. 2023 Apr;16(4):e013020. doi: 10.1161/CIRCINTERVENTIONS.123.013020. Epub 2023 Mar 28. Circ Cardiovasc Interv. 2023. PMID: 36974679 No abstract available.
References
-
- Jespersen L, Abildstrom SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–581. doi: 10.1007/s00392-013-0568-z - PubMed
-
- Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, et al. ; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963 - PubMed
-
- Al-Lamee RK, Shun-Shin MJ, Howard JP, Nowbar AN, Rajkumar C, Thompson D, Sen S, Nijjer S, Petraco R, Davies J, et al. Dobutamine stress echocardiography ischemia as a predictor of the placebo-controlled efficacy of percutaneous coronary intervention in stable coronary artery disease: the stress echocardiography-stratified analysis of ORBITA. Circulation. 2019;140:1971–1980. doi: 10.1161/CIRCULATIONAHA.119.042918 - PMC - PubMed
-
- Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE, Jr, Boden WE, Weintraub WS, et al. ; ISCHEMIA Research Group. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382:1408–1419. doi: 10.1056/NEJMoa1916370 - PMC - PubMed
-
- Stone GW, Ellis SG, Gori T, Metzger DC, Stein B, Erickson M, Torzewski J, Williams J, Jr, Lawson W, Broderick TM, et al. ; ABSORB IV Investigators. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1-year results from the ABSORB IV randomised trial. Lancet. 2018;392:1530–1540. doi: 10.1016/S0140-6736(18)32283-9 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

