Variable structure diversification by multicatalysis: the case of alcohols
- PMID: 36974691
- PMCID: PMC10111201
- DOI: 10.1039/d3cc00551h
Variable structure diversification by multicatalysis: the case of alcohols
Abstract
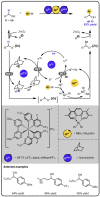
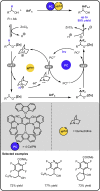
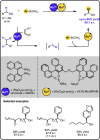
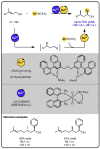
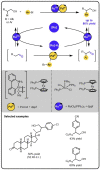
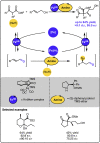
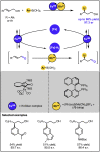
Given that alcohol moieties are present in a great diversity of valuable fine chemicals from nature and synthesis, methods enabling their structure diversification are highly sought after. Catalysis proved to enable the development of new transformations that are beyond the inherent reactivity of alcohols. However, modifying the structure of alcohols at certain unbiased positions remains a major challenge or requires tedious multistep procedures. Recently, increased attention has been given to multicatalyis, which combines multiple reactions and catalysts within one system, creating room for discovering previously inaccessible reactivities or increasing the overall efficiency of multistep transformations. This feature article focuses on demonstrating various aspects of devising such multicatalytic systems that modify the structure of alcohol-containing compounds. Special attention is given to highlighting the challenges and advantages of multicatalysis, and in a broader context discussing how the field of catalysis may progress toward more complex systems.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Clayden J., Greeves N., Warren S. and Wothers P., Organic Chemistry, Oxford University Press, New York, USA, 2001
Publication types
LinkOut - more resources
Full Text Sources

