ZBP1 Protects Against mtDNA-Induced Myocardial Inflammation in Failing Hearts
- PMID: 36974722
- PMCID: PMC10144299
- DOI: 10.1161/CIRCRESAHA.122.322227
ZBP1 Protects Against mtDNA-Induced Myocardial Inflammation in Failing Hearts
Abstract
Background: Mitochondrial DNA (mtDNA)-induced myocardial inflammation is intimately involved in cardiac remodeling. ZBP1 (Z-DNA binding protein 1) is a pattern recognition receptor positively regulating inflammation in response to mtDNA in inflammatory cells, fibroblasts, and endothelial cells. However, the role of ZBP1 in myocardial inflammation and cardiac remodeling remains unclear. The aim of this study was to elucidate the role of ZBP1 in mtDNA-induced inflammation in cardiomyocytes and failing hearts.
Methods: mtDNA was administrated into isolated cardiomyocytes. Myocardial infarctionwas conducted in wild type and ZBP1 knockout mice.
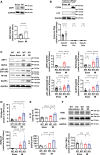
Results: We here found that, unlike in macrophages, ZBP1 knockdown unexpectedly exacerbated mtDNA-induced inflammation such as increases in IL (interleukin)-1β and IL-6, accompanied by increases in RIPK3 (receptor interacting protein kinase 3), phosphorylated NF-κB (nuclear factor-κB), and NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) in cardiomyocytes. RIPK3 knockdown canceled further increases in phosphorylated NF-κB, NLRP3, IL-1β, and IL-6 by ZBP1 knockdown in cardiomyocytes in response to mtDNA. Furthermore, NF-κB knockdown suppressed such increases in NLRP3, IL-1β, and IL-6 by ZBP1 knockdown in response to mtDNA. CpG-oligodeoxynucleotide, a Toll-like receptor 9 stimulator, increased RIPK3, IL-1β, and IL-6 and ZBP1 knockdown exacerbated them. Dloop, a component of mtDNA, but not Tert and B2m, components of nuclear DNA, was increased in cytosolic fraction from noninfarcted region of mouse hearts after myocardial infarction compared with control hearts. Consistent with this change, ZBP1, RIPK3, phosphorylated NF-κB, NLRP3, IL-1β, and IL-6 were increased in failing hearts. ZBP1 knockout mice exacerbated left ventricular dilatation and dysfunction after myocardial infarction, accompanied by further increases in RIPK3, phosphorylated NF-κB, NLRP3, IL-1β, and IL-6. In histological analysis, ZBP1 knockout increased interstitial fibrosis and myocardial apoptosis in failing hearts.
Conclusions: Our study reveals unexpected protective roles of ZBP1 against cardiac remodeling as an endogenous suppressor of mtDNA-induced myocardial inflammation.
Keywords: cytokines; fibrosis; heart failure; inflammation; macrophages; nucleotides; receptors, pattern recognition.
Conflict of interest statement
H. Tsutsui reports personal fees from Merck Sharp and Dohme (MSD), Astellas, Pfizer, Bristol-Myers Squibb, Otsuka Pharmaceutical, Daiichi-Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Kowa Pharmaceutical, Teijin Pharma, Medical Review Co, and Japanese Journal of Clinical Medicine; nonfinancial support from Actelion Pharmaceuticals, Japan Tobacco Inc, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Daiichi-Sankyo, IQVIA Services Japan, and Omron Healthcare Co; grants from Astellas, Novartis Pharma, Daiichi-Sankyo, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, and Teijin Pharma, MSD, outside the submitted work. The other authors report no conflicts.
Figures







References
-
- Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005 - PubMed
-
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055 - PubMed
-
- Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997 - PubMed
-
- Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451 - PubMed
-
- Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, et al. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111:1037–1047. doi: 10.1161/CIRCRESAHA.112.270132 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

