Circ_0022920 Maintains the Contractile Phenotype of Human Aortic Vascular Smooth Muscle Cells Via Sponging microRNA-650 and Promoting Transforming Growth Factor Beta Receptor 1 Expression in Angiotensin II-Induced Models for Aortic Dissection
- PMID: 36974747
- PMCID: PMC10122879
- DOI: 10.1161/JAHA.122.027425
Circ_0022920 Maintains the Contractile Phenotype of Human Aortic Vascular Smooth Muscle Cells Via Sponging microRNA-650 and Promoting Transforming Growth Factor Beta Receptor 1 Expression in Angiotensin II-Induced Models for Aortic Dissection
Abstract
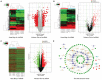
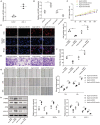
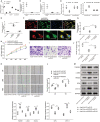
Background Abnormal regulation of vascular smooth muscle cells is regarded as the iconic pathological change of aortic dissection (AD). Herein, we aim to identify circ_0022920 as a crucial regulator in AD. Methods and Results Microarray analysis of circular RNAs, messenger RNAs, and micro RNAs in patients with AD was performed, and we identified that circ_0022920 was significantly downregulated in these patients. The Pearson correlation analysis uncovered the negative correlation between miR-650 and circ_0022920 or TGFβR1 (transforming growth factor beta receptor 1). Angiotensin II was used to treat human aortic vascular smooth muscle cells (HASMCs) and mice as models for AD. Hematoxylin and eosin and Masson's trichrome staining were used to analyze AD histopathology. Cell proliferation was analyzed with Cell Counting Kit-8 assay and EdU incorporation. Cell migration was assessed with transwell and wound healing assays. Enhanced circ_0022920 expression dramatically inhibited HASMC proliferation and migration and maintained contractile marker expression induced by angiotensin II, whereas miR-650 exerted opposite effects. MiR-650 was a target of circ_0022920. MiR-650 targeted IRF1 (interferon regulatory factor 1) and thus negatively regulated TGFβR1 expression to promote HASMC proliferation and migration and inhibit contractile marker expression. Circ_0022920 suppressed the progression of AD in vivo. Conclusions Circ_0022920 modulates the contractile phenotype of HASMCs via regulating the miR-650-IRF1-TGFβR1 axis in angiotensin II-induced models for AD, which provides potential therapeutic targets for AD.
Keywords: Circ_0022920; aortic dissection; contractile phenotype; miR‐650/IRF1/TGFβR1 axis.
Figures





References
-
- Juang D, Braverman AC, Eagle K. Cardiology patient pages. Aortic dissection. Circulation. 2008;118:e507–e510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

