A Novel Peptide-Based Detection of SARS-CoV-2 Antibodies
- PMID: 36975319
- PMCID: PMC10046560
- DOI: 10.3390/biomimetics8010089
A Novel Peptide-Based Detection of SARS-CoV-2 Antibodies
Abstract
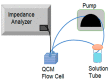
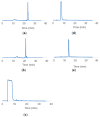
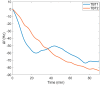
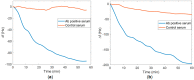
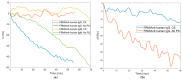
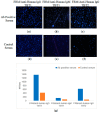
The need for rapidly developed diagnostic tests has gained significant attention after the recent pandemic. Production of neutralizing antibodies for vaccine development or antibodies to be used in diagnostic tests usually require the usage of recombinant proteins representing the infectious agent. However, peptides that can mimic these recombinant proteins may be rapidly utilized, especially in emergencies such as the recent outbreak. Here, we report two peptides that mimic the receptor binding domain of the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and investigate their binding behavior against the corresponding human immunoglobulin G and immunoglobulin M (IgG and IgM) antibodies in a clinical sample using a quartz crystal microbalance (QCM) sensor. These peptides were immobilized on a QCM sensor surface, and their binding behavior was studied against a clinical serum sample that was previously determined to be IgG and IgM-positive. It was determined that designed peptides bind to SARS-CoV-2 antibodies in a clinical sample. These peptides might be useful for the detection of SARS-CoV-2 antibodies using different methods such as enzyme-linked immunosorbent assay (ELISA) or lateral flow assays. A similar platform might prove to be useful for the detection and development of antibodies in other infections.
Keywords: SARS-CoV-2; antibody detection; biosensor; peptide mimetics; quartz crystal microbalance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Afzal A., Mujahid A., Schirhagl R., Bajwa S.Z., Latif U., Feroz S. Gravimetric viral diagnostics: Qcm based biosensors for early detection of viruses. Chemosens. 2017;5:7. doi: 10.3390/chemosensors5010007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

