CXCL1 and CXCL6 Are Potential Predictors for HCC Response to TACE
- PMID: 36975480
- PMCID: PMC10046993
- DOI: 10.3390/curroncol30030267
CXCL1 and CXCL6 Are Potential Predictors for HCC Response to TACE
Abstract
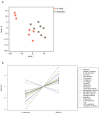
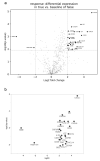
Distinct immune patterns of hepatocellular carcinoma (HCC) may have prognostic implications in the response to transarterial chemoembolization (TACE). Thus, we aimed to exploratively analyze tumor tissue of HCC patients who do or do not respond to TACE, and to identify novel prognostic biomarkers predictive of response to TACE. We retrospectively included 15 HCC patients who had three consecutive TACE between January 2019 and November 2019. Eight patients had a response while seven patients had no response to TACE. All patients had measurable disease according to mRECIST. Corresponding tumor tissue samples were processed for differential expression profiling using NanoString nCounter® PanCancer immune profiling panel. Immune-related pathways were broadly upregulated in TACE responders. The top differentially regulated genes were the upregulated CXCL1 (log2fc 4.98, Benjamini-Hochberg (BH)-p < 0.001), CXCL6 (log2fc 4.43, BH-p = 0.016) and the downregulated MME (log2fc -4.33, BH-p 0.001). CD8/T-regs was highly increased in responders, whereas the relative number of T-regs to tumor-infiltrating lymphocytes (TIL) was highly decreased. We preliminary identified CXCL1 and CXCL6 as candidate genes that might have the potential to serve as therapeutically relevant biomarkers in HCC patients. This might pave the way to improve patient selection for TACE in HCC patients beyond expert consensus.
Keywords: biomarker; hepatocellular carcinoma; immune profiling; transarterial chemoembolization.
Conflict of interest statement
H.R. was on an advisory board of Bristol-Myers Squibb; received honoraria from Roche, Bristol-Myers Squibb, Janssen-Cilag GmbH, Novartis, and Astra Zeneca; received travel support from Philips, Roche, and Bristol-Myers Squibb; received grants from Bristol-Myers Squibb; and holds shares of Bayer. F. F. has received travel support from Ipsen, and speaker fees from AbbVie, MSD, Ipsen, Eisai, and Fresenius. S.Z. has received speaker fees and/or honoraria for consultancy from AbbVie, Allergan, BioMarin, Gilead, Intercept, Janssen, MSD/Merck, NovoNordisk, SoBi, and Theratechnologies. P.J.W. has received consulting fees and honoraria for lectures by Bayer, Janssen-Cilag, Novartis, Roche, MSD, Astellas Pharma, Bristol-Myers Squibb, Thermo Fisher Scientific, Molecular Health, Guardant Health, Sophia Genetics, Qiagen, Eli Lilly, Myriad, Hedera Dx, and Astra Zeneca; research support was provided by Astra Zeneca. All other authors declare no conflicts of interest.
Figures

References
-
- Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- Gruber-Rouh T., Schmitt C., Naguib N.N.N., Nour-Eldin N.A., Eichler K., Beeres M., Vogl T.J. Transarterial chemoembolization (TACE) using mitomycin and lipiodol with or without degradable starch microspheres for hepatocellular carcinoma: Comparative study. BMC Cancer. 2018;18:188. doi: 10.1186/s12885-018-4099-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

