Bridging with surgically placed microaxial left ventricular assist devices: a high-volume centre experience
- PMID: 36975609
- PMCID: PMC10257579
- DOI: 10.1093/ejcts/ezad116
Bridging with surgically placed microaxial left ventricular assist devices: a high-volume centre experience
Abstract
Objectives: The Impella 5.0 and 5.5 have largely superseded non-ambulatory temporary mechanical support devices; yet, clinical outcomes are predominantly limited to small series: this study presents the experience of a high-volume centre.
Methods: An institutional clinical registry was used to identify all patients with cardiogenic shock who underwent Impella 5.0 or 5.5 implantation from January 2014 to March 2022. The primary outcome was survival to device explantation.
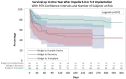
Results: The study cohort comprised 221 patients, including 146 (66.1%) Impella 5.0 and 75 (33.9%) Impella 5.5 patients. The primary aetiology was non-ischaemic cardiomyopathy (50.7%, n = 112), ischaemic cardiomyopathy (23.1%, n = 51) and acute myocardial infarction (26.2%, n = 58). Patients were prospectively classified according to strategy as bridge to transplant (47.5%, n = 105), bridge to durable device (13.6%, n = 30) or bridge to recovery (38.9%, n = 86). Patients were predominantly Interagency Registry for Mechanically Assisted Circulatory Support profile 1 or 2 (95.0%, n = 210). The median bridging duration was 14 (range 0-137) days. Device exchange, Ischaemic stroke and ipsilateral arm ischaemia occurred in 8.1% (n = 18), 2.7% (n = 6) and 1.8% (n = 4) of patients, respectively. Compared to the 75 most recent Impella 5.0 patients, Impella 5.5 patients (n = 75) had lower rates of device exchange (4.0%, n = 3 vs 13.3%, n = 10, P = 0.04). Overall, 70.1% (n = 155) of patients survived to Impella explantation.
Conclusions: The Impella 5.0 and 5.5 provide safe and effective temporary mechanical support in appropriately selected patients with cardiogenic shock. The newer device generation may have a lower requirement for device exchange as compared to its predecessor.
Keywords: Heart transplant; heart failure; mechanical circulatory support.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved.
Figures

Comment in
-
Bridging with surgical implanted Impella devices.Eur J Cardiothorac Surg. 2023 Jun 1;63(6):ezad213. doi: 10.1093/ejcts/ezad213. Eur J Cardiothorac Surg. 2023. PMID: 37233201 No abstract available.
References
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM et al 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. - PubMed
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. - PubMed
-
- Sassard T, Scalabre A, Bonnefoy E, Sanchez I, Farhat F, Jegaden O.. The right axillary artery approach for the Impella Recover LP 5.0 microaxial pump. Ann Thorac Surg 2008;85:1468–70. - PubMed
-
- Stretch R, Sauer CM, Yuh DD, Bonde P.. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407–15. - PubMed
-
- Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ et al National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention 2018;13:e2152–e2159. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

