Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics
- PMID: 36975627
- PMCID: PMC10048531
- DOI: 10.3390/gels9030178
Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics
Abstract
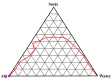
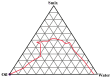
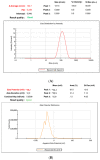
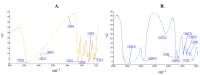
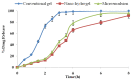
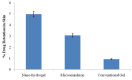
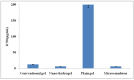
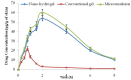
Mangiferin is a herbal drug that has proven anticancer potential. Owing to its lower aqueous solubility and poor oral bioavailability, the full pharmacological potential of this bioactive drug has not fully been explored. In the present study, phospholipid-based microemulsion systems were developed to bypass oral delivery. The globule size of the developed nanocarriers was less than 150 nm and the drug entrapment was >75% with a drug loading ~25%. The developed system offered a controlled release pattern following the Fickian drug release. This enhanced mangiferin's in vitro anticancer activity by four-fold, the cellular uptake was observed to be improved by three-fold on the MCF-7 cells. Ex vivo dermatokinetic studies showed substantial topical bioavailability with a prolonged residence time. The findings provide a simple technique to administer mangiferin via a topical route promising a safer, topically bioavailable and effective treatment option for breast cancer. Such scalable carriers with immense topical delivery potential may provide a better option for present-day topical products of a conventional nature.
Keywords: anticancer; breast cancer; dermal bioavailability; dermal pharmacokinetics; microemulsion; nanoemulsion; topical administration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yap K.M., Sekar M., Seow L.J., Gan S.H., Bonam S.R., Rani N.N.I.M., Lum P.T., Subramaniyan V., Wu Y.S., Fuloria N.K., et al. Mangifera indica (Mango): A Promising Medicinal Plant for Breast Cancer Therapy and Understanding Its Potential Mechanisms of Action. Breast Cancer Targets Ther. 2021;13:471–503. doi: 10.2147/BCTT.S316667. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

