Formation of Alginate/Chitosan Interpenetrated Networks Revealed by EPR Spectroscopy
- PMID: 36975680
- PMCID: PMC10048464
- DOI: 10.3390/gels9030231
Formation of Alginate/Chitosan Interpenetrated Networks Revealed by EPR Spectroscopy
Abstract
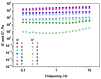
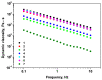
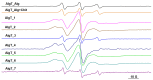
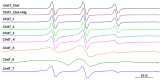
This study analyzes the physico-chemical properties of interpenetrated polymer networks (IPNs) and semi-IPN resulting from cross-linking chitosan with glutaraldehyde and alginate with Ca2+ cations, as a function of the order in which the cross-linking agents are added to the polymer mixture. Three physico-chemical methods were used to assess the differences between systems: rheology, IR spectroscopy, and electron paramagnetic resonance (EPR) spectroscopy. While rheology and IR spectroscopy are commonly used to characterize gel materials, EPR spectroscopy is rarely used, but has the advantage of providing local information about the dynamics of a system. The rheological parameters, which describe the global behavior of the samples, show that semi-IPN systems have a weaker gel behavior and the order of introducing the cross-linker in the polymer systems plays a role. The IR spectra of samples resulting by adding only Ca2+ or Ca2+ as the first cross-linker are similar to that of the alginate gel, while the spectra of samples in which glutaraldehyde is firstly added resemble the chitosan gel spectrum. Using spin-labeled alginate and spin-labeled chitosan, we monitored the changes occurring in the dynamic of the spin labels due to the formation of IPN and semi-IPN. The results show that the order of adding the cross-linking agents influences the dynamic of the IPN network, and that the formation of the alginate network determines the characteristics of the entire IPN system. The EPR data were correlated with the rheological parameters and IR spectra of the analyzed samples.
Keywords: EPR spectroscopy; IPN; IR spectroscopy; rheology; spin-labeled alginate; spin-labeled chitosan.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
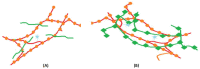


References
-
- Millar J.R. Interpenetrating polymer networks. Styrene-divinylbenzenecopolymers with two and three interpenetrating networks, and their sulphonates. J. Chem. Soc. 1960:1311–1317. doi: 10.1039/JR9600001311. - DOI
-
- Roland C.M. Interpenetrating polymer networks (IPN): Structure and mechanical behavior. In: Kobayashi S., Müllen K., editors. Encyclopedia of Polymeric Nanomaterials. Springer; Berlin/Heidelberg, Germany: 2013. pp. 1–9. - DOI
-
- Sperling L.H. Interpenetrating polymer networks: An overview. In: Klempner D., Sperling L.H., Utracki L.A., editors. Interpenetrating Polymer Networks (ACS Advances in Chemistry Volume 239) American Chemical Society; Washington, DC, USA: 1994. pp. 3–38. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

