Tuberculostearic Acid Controls Mycobacterial Membrane Compartmentalization
- PMID: 36976029
- PMCID: PMC10127668
- DOI: 10.1128/mbio.03396-22
Tuberculostearic Acid Controls Mycobacterial Membrane Compartmentalization
Abstract
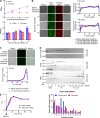
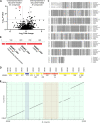
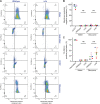
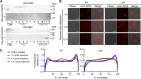
The intracellular membrane domain (IMD) is a laterally discrete region of the mycobacterial plasma membrane, enriched in the subpolar region of the rod-shaped cell. Here, we report genome-wide transposon sequencing to discover the controllers of membrane compartmentalization in Mycobacterium smegmatis. The putative gene cfa showed the most significant effect on recovery from membrane compartment disruption by dibucaine. Enzymatic analysis of Cfa and lipidomic analysis of a cfa deletion mutant (Δcfa) demonstrated that Cfa is an essential methyltransferase for the synthesis of major membrane phospholipids containing a C19:0 monomethyl-branched stearic acid, also known as tuberculostearic acid (TBSA). TBSA has been intensively studied due to its abundant and genus-specific production in mycobacteria, but its biosynthetic enzymes had remained elusive. Cfa catalyzed the S-adenosyl-l-methionine-dependent methyltransferase reaction using oleic acid-containing lipid as a substrate, and Δcfa accumulated C18:1 oleic acid, suggesting that Cfa commits oleic acid to TBSA biosynthesis, likely contributing directly to lateral membrane partitioning. Consistent with this model, Δcfa displayed delayed restoration of subpolar IMD and delayed outgrowth after bacteriostatic dibucaine treatment. These results reveal the physiological significance of TBSA in controlling lateral membrane partitioning in mycobacteria. IMPORTANCE As its common name implies, tuberculostearic acid is an abundant and genus-specific branched-chain fatty acid in mycobacterial membranes. This fatty acid, 10-methyl octadecanoic acid, has been an intense focus of research, particularly as a diagnostic marker for tuberculosis. It was discovered in 1934, and yet the enzymes that mediate the biosynthesis of this fatty acid and the functions of this unusual fatty acid in cells have remained elusive. Through a genome-wide transposon sequencing screen, enzyme assay, and global lipidomic analysis, we show that Cfa is the long-sought enzyme that is specifically involved in the first step of generating tuberculostearic acid. By characterizing a cfa deletion mutant, we further demonstrate that tuberculostearic acid actively regulates lateral membrane heterogeneity in mycobacteria. These findings indicate the role of branched fatty acids in controlling the functions of the plasma membrane, a critical barrier for the pathogen to survive in its human host.
Keywords: fatty acids; lipidomics; membrane domain; phospholipids; transposon sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

