Metabolic dysfunction-associated fatty liver disease (MAFLD): an update of the recent advances in pharmacological treatment
- PMID: 36976456
- PMCID: PMC10635944
- DOI: 10.1007/s13105-023-00954-4
Metabolic dysfunction-associated fatty liver disease (MAFLD): an update of the recent advances in pharmacological treatment
Abstract
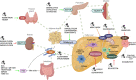
Metabolic dysfunction-associated fatty liver disease (MAFLD) is nowadays considered the liver manifestation of metabolic syndrome. Its prevalence is increasing worldwide in parallel to the epidemic of diabetes and obesity. MAFLD includes a wide spectrum of liver injury including simple steatosis and non-alcoholic steatohepatitis (NASH) that may lead to serious complications such as liver cirrhosis and liver cancer. The complexity of its pathophysiology and the intricate mechanisms underlying disease progression explains the huge variety of molecules targeting diverse biological mechanisms that have been tested in preclinical and clinical settings in the last two decades. Thanks to the large number of clinical trials of the last few years, most of them still ongoing, the pharmacotherapy scenario of MAFLD is rapidly evolving. The three major components of MAFLD, steatosis, inflammation, and fibrosis seem to be safely targeted with different agents at least in a large proportion of patients. Likely, in the next few years more than one drug will be approved for the treatment of MAFLD at different disease stages. The aim of this review is to synthesize the characteristics and the results of the most advanced clinical trials for the treatment of NASH to evaluate the recent advances of pharmacotherapy in this disease.
Keywords: Clinical trials; Drug therapies; MAFLD; NAFLD; NASH.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. The Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
-
- WHO [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical