Parameters Associated with the Required Drug Dose of Intravenous Immunoglobulin in Stable Chronic Inflammatory Demyelinating Polyradiculoneuropathy
- PMID: 36976670
- PMCID: PMC10058913
- DOI: 10.3390/neurolint15010027
Parameters Associated with the Required Drug Dose of Intravenous Immunoglobulin in Stable Chronic Inflammatory Demyelinating Polyradiculoneuropathy
Abstract
Background: Intravenous immunoglobulin (IVIg) is efficient and one of very few treatment options for patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). However, finding the optimal dose of IVIg for individual CIDP patients remains challenging. The dose of IVIg needs to be adjusted individually. Considering the high healthcare costs of IVIg therapy, the overtreatment of some patients seen in placebo studies and the shortage of IVIg we recently experienced, as well as identifying factors associated with the required dose of IVIg in maintenance treatment, is extremely important. Thus, in this retrospective study, we analyze characteristics of patients with stable CIDP, which are associated with the required drug dose.
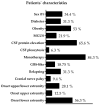
Methods: 32 patients with stable CIDP treated with IVIg between July 2021 and July 2022 were identified from our database and included in this retrospective study. Patients' characteristics were registered, and parameters were identified that were associated with the IVIg dose.
Results: Age, cerebrospinal fluid protein elevation, disease duration, delay between symptom onset/diagnosis, Inflammatory Neuropathy Cause and Treatment (INCAT) score, and Medical Research Council Sum Score (MRC SS) were significantly associated with the required drug dose. In addition, an association of age, sex, elevated CSF protein, time interval between symptom onset and diagnosis, and the MRC SS with the required IVIg dose could be demonstrated in the multivariable regression analysis.
Conclusions: Our model, which is based on routine parameters that are simple to address in the clinical practice, can be useful in adjusting the IVIg dose in patients with stable CIDP.
Keywords: CIDP; IVIg; dosing; maintenance therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Doneddu P.E., Cocito D., Manganelli F., Fazio R., Briani C., Filosto M., Benedetti L., Mazzeo A., Marfia G.A., Cortese A., et al. Atypical CIDP: Diagnostic criteria, progression and treatment response. Data from the Italian CIDP Database. J. Neurol. Neurosurg. Psychiatry. 2019;90:125–132. doi: 10.1136/jnnp-2018-318714. - DOI - PubMed
-
- Bergh P.Y.K.V.D., Doorn P.A., Hadden R.D.M., Avau B., Vankrunkelsven P., Allen J.A., Attarian S., Blomkwist-Markens P.H., Cornblath D.R., Eftimov F., et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. Eur. J. Neurol. 2021;28:3556–3583. doi: 10.1111/ene.14959. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

