Pattern dynamics and stochasticity of the brain rhythms
- PMID: 36976768
- PMCID: PMC10083604
- DOI: 10.1073/pnas.2218245120
Pattern dynamics and stochasticity of the brain rhythms
Abstract
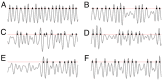
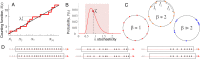
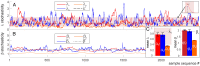
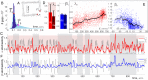
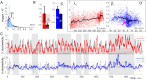
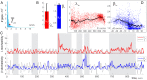
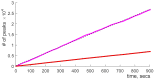
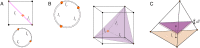
Our current understanding of brain rhythms is based on quantifying their instantaneous or time-averaged characteristics. What remains unexplored is the actual structure of the waves-their shapes and patterns over finite timescales. Here, we study brain wave patterning in different physiological contexts using two independent approaches: The first is based on quantifying stochasticity relative to the underlying mean behavior, and the second assesses "orderliness" of the waves' features. The corresponding measures capture the waves' characteristics and abnormal behaviors, such as atypical periodicity or excessive clustering, and demonstrate coupling between the patterns' dynamics and the animal's location, speed, and acceleration. Specifically, we studied patterns of θ, γ, and ripple waves recorded in mice hippocampi and observed speed-modulated changes of the wave's cadence, an antiphase relationship between orderliness and acceleration, as well as spatial selectiveness of patterns. Taken together, our results offer a complementary-mesoscale-perspective on brain wave structure, dynamics, and functionality.
Keywords: hippocampus; learning and memory; patterning; waveforms.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Buzsáki G., Rhythms in the Brain (Oxford University Press, New York, 2011).
-
- Skaggs W., McNaughton B., Wilson M., Barnes C., Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 (1996). - PubMed
-
- Bench K., et al. , Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936 (2010). - PubMed
-
- Nikoli D., Fries P., Singer W., Gamma oscillations: Precise temporal coordination without a metronome. Trends Cogn. Sci. 17, 54–55 (2013). - PubMed
-
- Colgin L., et al. , Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

