Longitudinal temperature measurement can determine humane endpoints in BALB/c mouse models of ESKAPEE infection
- PMID: 36976806
- PMCID: PMC10054282
- DOI: 10.1080/21505594.2023.2186331
Longitudinal temperature measurement can determine humane endpoints in BALB/c mouse models of ESKAPEE infection
Abstract
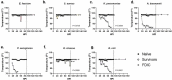
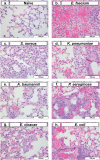
Antimicrobial resistance (AMR) is a worldwide problem, which is driving more preclinical research to find new treatments and countermeasures for drug-resistant bacteria. However, translational models in the preclinical space have remained static for years. To improve animal use ethical considerations, we assessed novel methods to evaluate survival after lethal infection with ESKAPEE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter cloacae, and Escherichia coli) in pulmonary models of infection. Consistent with published lung infection models often used for novel antimicrobial development, BALB/c mice were immunosuppressed with cyclophosphamide and inoculated intranasally with individual ESKAPEE pathogens or sterile saline. Observations were recorded at frequent intervals to determine predictive thresholds for humane endpoint decision-making. Internal temperature was measured via implanted IPTT300 microchips, and external temperature was measured using a non-contact, infrared thermometer. Additionally, clinical scores were evaluated based on animal appearance, behaviour, hydration status, respiration, and body weight. Internal temperature differences between survivors and non-survivors were statistically significant for E. faecium, S. aureus, K. pneumoniae, A. baumannii, E. cloacae, and E. coli, and external temperature differences were statistically significant for S. aureus, K. pneumoniae, E. cloacae, and E. coli. Internal temperature more precisely predicted mortality compared to external temperature, indicating that a threshold of 85ºF (29.4ºC) was 86.0% predictive of mortality and 98.7% predictive of survival. Based on our findings, we recommend future studies involving BALB/c mice ESKAPEE pathogen infection use temperature monitoring as a humane endpoint threshold.
Keywords: BALB/c; ESKAPEE; Temperature; humane endpoints; murine pulmonary infection model; replacement, reduction, refinement (3Rs).
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
