Hypoxia-Induced Kidney Injury in Newborn Rats
- PMID: 36977025
- PMCID: PMC10053593
- DOI: 10.3390/toxics11030260
Hypoxia-Induced Kidney Injury in Newborn Rats
Abstract
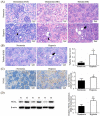
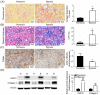
Exposure to hypoxia during the early postnatal period can have adverse effects on vital organs. Neonatal Sprague-Dawley rats housed in a hypoxic chamber were compared to those in a normoxic chamber from postnatal days 0 to 7. Arterial blood was collected to evaluate renal function and hypoxia. Kidney morphology and fibrosis were evaluated using staining methods and immunoblotting. In the kidneys of the hypoxic group, protein expressions of hypoxia-inducible factor-1 were higher than those in the normoxic group. Hypoxic rats had higher levels of hematocrit, serum creatinine, and lactate than normoxic rats. Body weight was reduced, and protein loss of kidney tissue was observed in hypoxic rats compared to normoxic rats. Histologically, hypoxic rats showed glomerular atrophy and tubular injury. Renal fibrosis with collagen fiber deposition was observed in the hypoxic group. The expression of nicotinamide adenine dinucleotide phosphate oxidases was enhanced in the kidneys of hypoxic rats. Proteins involved in apoptosis were upregulated in the kidneys of hypoxic rats. An increase in the expression of pro-inflammatory cytokines was also observed in the kidneys of hypoxic rats. Hypoxic kidney injury in neonatal rats was associated with oxidative stress, inflammation, apoptosis, and fibrosis.
Keywords: apoptosis; fibrosis; hypoxia; kidney injury; neonate; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

