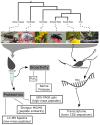
Functional and Proteomic Insights into Aculeata Venoms
- PMID: 36977115
- PMCID: PMC10053895
- DOI: 10.3390/toxins15030224
Functional and Proteomic Insights into Aculeata Venoms
Abstract
Aculeate hymenopterans use their venom for a variety of different purposes. The venom of solitary aculeates paralyze and preserve prey without killing it, whereas social aculeates utilize their venom in defence of their colony. These distinct applications of venom suggest that its components and their functions are also likely to differ. This study investigates a range of solitary and social species across Aculeata. We combined electrophoretic, mass spectrometric, and transcriptomic techniques to characterize the compositions of venoms from an incredibly diverse taxon. In addition, in vitro assays shed light on their biological activities. Although there were many common components identified in the venoms of species with different social behavior, there were also significant variations in the presence and activity of enzymes such as phospholipase A2s and serine proteases and the cytotoxicity of the venoms. Social aculeate venom showed higher presence of peptides that cause damage and pain in victims. The venom-gland transcriptome from the European honeybee (Apis mellifera) contained highly conserved toxins which match those identified by previous investigations. In contrast, venoms from less-studied taxa returned limited results from our proteomic databases, suggesting that they contain unique toxins.
Keywords: Aculeata; cytotoxicity; proteomics; sociality; venom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

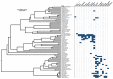
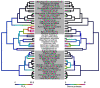
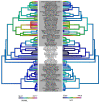
 , and amino acids are colored according to the default settings of AliView [79]. Toxin families include: (A) icarapins, (B) phospholipase A2, (C) anthophilins such as apamin [78], (D) carboxylesterases. (E) Relative length-normalized expression of these toxin families in the transcriptome, measured as total RPK for each family.
, and amino acids are colored according to the default settings of AliView [79]. Toxin families include: (A) icarapins, (B) phospholipase A2, (C) anthophilins such as apamin [78], (D) carboxylesterases. (E) Relative length-normalized expression of these toxin families in the transcriptome, measured as total RPK for each family.
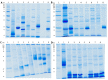

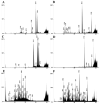
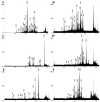
References
-
- Piek T. Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects. Academic Press; London, UK: 1986.
-
- Gaston K.J. The magnitude of global insect species richness. Conserv. Biol. 1991;5:283–296. doi: 10.1111/j.1523-1739.1991.tb00140.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

