A Calcium- and GTP-Dependent Transglutaminase in Leishmania infantum
- PMID: 36977273
- PMCID: PMC10053793
- DOI: 10.3390/vetsci10030234
A Calcium- and GTP-Dependent Transglutaminase in Leishmania infantum
Abstract
While human and animal leishmaniasis affect several millions of people worldwide, L. infantum is the species responsible for visceral leishmaniasis in Europe, Middle East, and America. Antileishmanial drugs present issues associated with drug toxicity and increasing parasite resistance. Therefore, the study of this parasite with a focus on new potential drug targets is extremely useful. Accordingly, we purified and characterized a transglutaminase (TGase) from L. infantum promastigotes. While Tgases are known to be involved in cell death and autophagy, it appears that these functions are very important for parasites' virulence. For the first time, we showed a Ca2+- and GTP-dependent TGase in Leishmania corresponding to a 54 kDa protein, which was purified by two chromatographic steps: DEAE-Sepharose and Heparin-Sepharose. Using polyclonal antibodies against a 50-amino-acid conserved region of the catalytic core of human TGase 2, we revealed two other bands of 66 and 75 kDa. The 54 kDa band appears to be different from the previously reported TGase, which was shown to be Ca2+- independent. Future research should address the identification of the purified enzyme sequence and, subsequently, its cloning to more comprehensively investigate its pathophysiological function and possible differences from mammal enzymes.
Keywords: GTP-dependent activity; Leishmania; calcium-dependent activity; protein cross-linking; transamidation; transglutaminase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

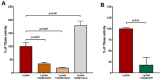
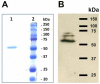

References
-
- Bruschi F., Gradoni L. The Leishmaniases: Old Neglected Tropical Diseases. Springer International Publishing; Cham, Switzerland: 2018.
LinkOut - more resources
Full Text Sources
Miscellaneous

