Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial
- PMID: 36977309
- PMCID: PMC10414746
- DOI: 10.1200/JCO.22.02558
Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial
Abstract
Purpose: To investigate the efficacy and safety of trastuzumab deruxtecan, an antibody-drug conjugate targeting human epidermal growth factor receptor 2 (HER2) with a topoisomerase I inhibitor payload, in patients with uterine carcinosarcoma (UCS) expressing HER2.
Patients and methods: Patients with recurrent UCS with HER2 immunohistochemistry scores ≥1+ previously treated with chemotherapy were included. Patients were assigned to the HER2-high (immunohistochemistry score ≥2+; n = 22) or low (immunohistochemistry score of 1+; n = 10) groups for primary and exploratory analyses, respectively. Trastuzumab deruxtecan 6.4 or 5.4 mg/kg was administered intravenously once every 3 weeks until unacceptable toxicity or disease progression. Dose modification was based on the updated recommended phase II dose for breast cancer to be 5.4 mg/kg. The primary end point was the objective response rate by central review in the HER2-high group. Secondary end points included the overall response rate (ORR) in the HER2-high group by investigator assessment, ORR in the HER2-low group, progression-free survival (PFS), overall survival (OS), and safety.
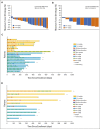
Results: The ORR by central review in the HER2-high and HER2-low groups were 54.5% (95% CI, 32.2 to 75.6) and 70.0% (95% CI, 34.8 to 93.3) and those by investigator assessments were 68.2% and 60.0%, respectively. The median PFS and OS in the HER2-high and HER2-low groups were 6.2 and 13.3 months and 6.7 months and not reached, respectively. Grade ≥ 3 adverse events occurred in 20 patients (61%). Grades 1-2 and 3 pneumonitis/interstitial lung disease occurred in eight (24%) and one (3%) patient, respectively.
Conclusion: Trastuzumab deruxtecan has efficacy in patients with UCS, regardless of HER2 status. The safety profile was generally consistent with that previously reported. Toxicities were manageable with appropriate monitoring and treatment.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Matsuzaki S, Klar M, Matsuzaki S, et al. : Uterine carcinosarcoma: Contemporary clinical summary, molecular updates, and future research opportunity. Gynecol Oncol 160:586-601, 2021 - PubMed
-
- Jonson AL, Bliss RL, Truskinovsky A, et al. : Clinical features and outcomes of uterine and ovarian carcinosarcoma. Gynecol Oncol 100:561-564, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

