Single Systemic Administration of a Gene Therapy Leading to Disease Treatment in Metachromatic Leukodystrophy Arsa Knock-Out Mice
- PMID: 36977578
- PMCID: PMC10184740
- DOI: 10.1523/JNEUROSCI.1829-22.2023
Single Systemic Administration of a Gene Therapy Leading to Disease Treatment in Metachromatic Leukodystrophy Arsa Knock-Out Mice
Abstract
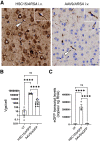
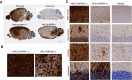
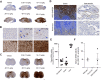
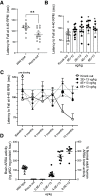
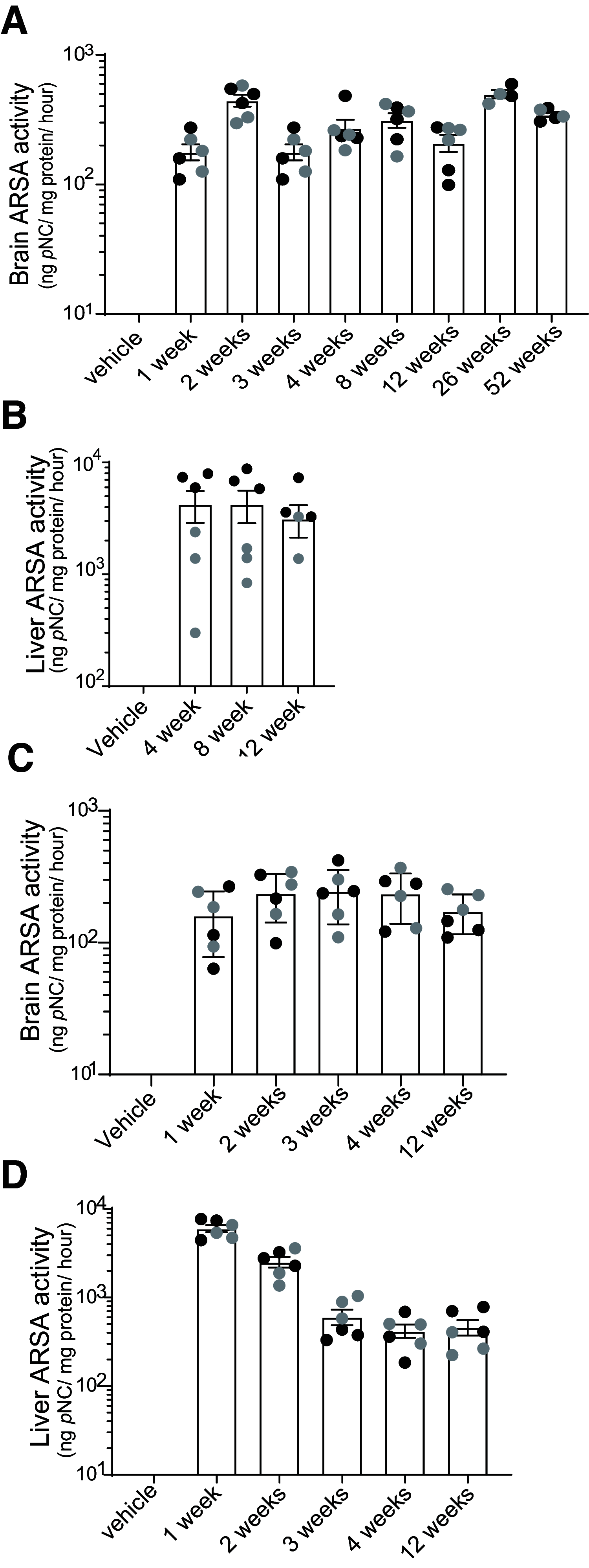
Metachromatic leukodystrophy (MLD) is a rare, inherited, demyelinating lysosomal storage disorder caused by mutations in the arylsulfatase-A gene (ARSA). In patients, levels of functional ARSA enzyme are diminished and lead to deleterious accumulation of sulfatides. Herein, we demonstrate that intravenous administration of HSC15/ARSA restored the endogenous murine biodistribution of the corresponding enzyme, and overexpression of ARSA corrected disease biomarkers and ameliorated motor deficits in Arsa KO mice of either sex. In treated Arsa KO mice, when compared with intravenously administered AAV9/ARSA, significant increases in brain ARSA activity, transcript levels, and vector genomes were observed with HSC15/ARSA Durability of transgene expression was established in neonate and adult mice out to 12 and 52 weeks, respectively. Levels and correlation between changes in biomarkers and ARSA activity required to achieve functional motor benefit was also defined. Finally, we demonstrated blood-nerve, blood-spinal and blood-brain barrier crossing as well as the presence of circulating ARSA enzyme activity in the serum of healthy nonhuman primates of either sex. Together, these findings support the use of intravenous delivery of HSC15/ARSA-mediated gene therapy for the treatment of MLD.SIGNIFICANCE STATEMENT Herein, we describe the method of gene therapy adeno-associated virus (AAV) capsid and route of administration selection leading to an efficacious gene therapy in a mouse model of metachromatic leukodystrophy. We demonstrate the therapeutic outcome of a new naturally derived clade F AAV capsid (AAVHSC15) in a disease model and the importance of triangulating multiple end points to increase the translation into higher species via ARSA enzyme activity and biodistribution profile (with a focus on the CNS) with that of a key clinically relevant biomarker.
Keywords: AAVHSC; biomarker; gene therapy; metachromatic leukodystrophy; motor deficit; translation.
Copyright © 2023 the authors.
Figures

References
-
- Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE (1991) Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med 324:1464–1470. - PubMed
-
- Belur LR, Podetz-Pedersen KM, Tran TA, Mesick JA, Singh NM, Riedl M, Vulchanova L, Kozarsky KF, McIvor RS (2020) Intravenous delivery for treatment of mucopolysaccharidosis type I: a comparison of AAV serotypes 9 and rh10. Mol Genet Metab Rep 24:100604. 10.1016/j.ymgmr.2020.100604 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
