Domain-specific p53 mutants activate EGFR by distinct mechanisms exposing tissue-independent therapeutic vulnerabilities
- PMID: 36977662
- PMCID: PMC10050071
- DOI: 10.1038/s41467-023-37223-3
Domain-specific p53 mutants activate EGFR by distinct mechanisms exposing tissue-independent therapeutic vulnerabilities
Abstract
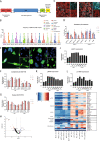
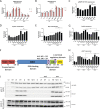
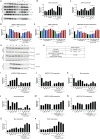
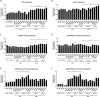
Mis-sense mutations affecting TP53 promote carcinogenesis both by inactivating tumor suppression, and by conferring pro-carcinogenic activities. We report here that p53 DNA-binding domain (DBD) and transactivation domain (TAD) mis-sense mutants unexpectedly activate pro-carcinogenic epidermal growth factor receptor (EGFR) signaling via distinct, previously unrecognized molecular mechanisms. DBD- and TAD-specific TP53 mutants exhibited different cellular localization and induced distinct gene expression profiles. In multiple tissues, EGFR is stabilized by TAD and DBD mutants in the cytosolic and nuclear compartments respectively. TAD mutants promote EGFR-mediated signaling by enhancing EGFR interaction with AKT via DDX31 in the cytosol. Conversely, DBD mutants maintain EGFR activity in the nucleus, by blocking EGFR interaction with the phosphatase SHP1, triggering c-Myc and Cyclin D1 upregulation. Our findings suggest that p53 mutants carrying gain-of-function, mis-sense mutations affecting two different domains form new protein complexes that promote carcinogenesis by enhancing EGFR signaling via distinctive mechanisms, exposing clinically relevant therapeutic vulnerabilities.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

