Mitochondria-derived H2O2 triggers liver regeneration via FoxO3a signaling pathway after partial hepatectomy in mice
- PMID: 36977674
- PMCID: PMC10050396
- DOI: 10.1038/s41419-023-05744-w
Mitochondria-derived H2O2 triggers liver regeneration via FoxO3a signaling pathway after partial hepatectomy in mice
Abstract
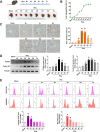
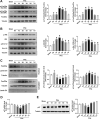
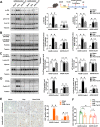
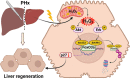
Reactive oxygen species (ROS) can induce oxidative injury and are generally regarded as toxic byproducts, although they are increasingly recognized for their signaling functions. Increased ROS often accompanies liver regeneration (LR) after liver injuries, however, their role in LR and the underlying mechanism remains unclear. Here, by employing a mouse LR model of partial hepatectomy (PHx), we found that PHx induced rapid increases of mitochondrial hydrogen peroxide (H2O2) and intracellular H2O2 at an early stage, using a mitochondria-specific probe. Scavenging mitochondrial H2O2 in mice with liver-specific overexpression of mitochondria-targeted catalase (mCAT) decreased intracellular H2O2 and compromised LR, while NADPH oxidases (NOXs) inhibition did not affect intracellular H2O2 or LR, indicating that mitochondria-derived H2O2 played an essential role in LR after PHx. Furthermore, pharmacological activation of FoxO3a impaired the H2O2-triggered LR, while liver-specific knockdown of FoxO3a by CRISPR-Cas9 technology almost abolished the inhibition of LR by overexpression of mCAT, demonstrating that FoxO3a signaling pathway mediated mitochondria-derived H2O2 triggered LR after PHx. Our findings uncover the beneficial roles of mitochondrial H2O2 and the redox-regulated underlying mechanisms during LR, which shed light on potential therapeutic interventions for LR-related liver injury. Importantly, these findings also indicate that improper antioxidative intervention might impair LR and delay the recovery of LR-related diseases in clinics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

