Correlative light-electron microscopy using small gold nanoparticles as single probes
- PMID: 36977682
- PMCID: PMC10050153
- DOI: 10.1038/s41377-023-01115-4
Correlative light-electron microscopy using small gold nanoparticles as single probes
Abstract
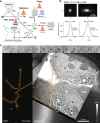
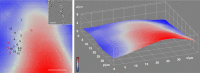
Correlative light-electron microscopy (CLEM) requires the availability of robust probes which are visible both in light and electron microscopy. Here we demonstrate a CLEM approach using small gold nanoparticles as a single probe. Individual gold nanoparticles bound to the epidermal growth factor protein were located with nanometric precision background-free in human cancer cells by light microscopy using resonant four-wave mixing (FWM), and were correlatively mapped with high accuracy to the corresponding transmission electron microscopy images. We used nanoparticles of 10 nm and 5 nm radius, and show a correlation accuracy below 60 nm over an area larger than 10 µm size, without the need for additional fiducial markers. Correlation accuracy was improved to below 40 nm by reducing systematic errors, while the localisation precision is below 10 nm. Polarisation-resolved FWM correlates with nanoparticle shapes, promising for multiplexing by shape recognition in future applications. Owing to the photostability of gold nanoparticles and the applicability of FWM microscopy to living cells, FWM-CLEM opens up a powerful alternative to fluorescence-based methods.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Muller-Reichert, T. & Verkade, P. Correlative light and electron microscopy. In Methods in Cell Biology (Elsevier, Academic Press, Cambridge MA, United States, 2021, Series Editors: Leslie Wilson and Phong Tran).
Grants and funding
- EP/I005072/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- EP/I016260/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- EP/L001470/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- EP/M028313/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- BBL014181/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
LinkOut - more resources
Full Text Sources

