Immunogenic hypofractionated radiotherapy sensitising head and neck squamous cell carcinoma to anti-PD-L1 therapy in MDSC-dependent manner
- PMID: 36977825
- PMCID: PMC10206106
- DOI: 10.1038/s41416-023-02230-0
Immunogenic hypofractionated radiotherapy sensitising head and neck squamous cell carcinoma to anti-PD-L1 therapy in MDSC-dependent manner
Abstract
Background: Enhancing the response rate of immunotherapy will aid in the success of cancer treatment. Here, we aimed to explore the combined effect of immunogenic radiotherapy with anti-PD-L1 treatment in immunotherapy-resistant HNSCC mouse models.
Methods: The SCC7 and 4MOSC2 cell lines were irradiated in vitro. SCC7-bearing mice were treated with hypofractionated or single-dose radiotherapy followed by anti-PD-L1 therapy. The myeloid-derived suppressive cells (MDSCs) were depleted using an anti-Gr-1 antibody. Human samples were collected to evaluate the immune cell populations and ICD markers.
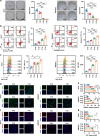
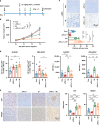
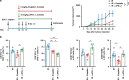
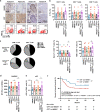
Results: Irradiation increased the release of immunogenic cell death (ICD) markers (calreticulin, HMGB1 and ATP) in SCC7 and 4MOSC2 in a dose-dependent manner. The supernatant from irradiated cells upregulated the expression of PD-L1 in MDSCs. Mice treated with hypofractionated but not single-dose radiotherapy were resistant to tumour rechallenge by triggering ICD, when combined with anti-PD-L1 treatment. The therapeutic efficacy of combination treatment partially relies on MDSCs. The high expression of ICD markers was associated with activation of adaptive immune responses and a positive prognosis in HNSCC patients.
Conclusion: These results present a translatable method to substantially improve the antitumor immune response by combining PD-L1 blockade with immunogenic hypofractionated radiotherapy in HNSCC.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells.Oral Oncol. 2021 Oct;121:105472. doi: 10.1016/j.oraloncology.2021.105472. Epub 2021 Jul 30. Oral Oncol. 2021. PMID: 34333450
-
A photodynamically sensitized dendritic cell vaccine that promotes the anti-tumor effects of anti-PD-L1 monoclonal antibody in a murine model of head and neck squamous cell carcinoma.J Transl Med. 2022 Nov 3;20(1):505. doi: 10.1186/s12967-022-03707-x. J Transl Med. 2022. PMID: 36329529 Free PMC article.
-
PD-L1-specific helper T-cells exhibit effective antitumor responses: new strategy of cancer immunotherapy targeting PD-L1 in head and neck squamous cell carcinoma.J Transl Med. 2019 Jun 20;17(1):207. doi: 10.1186/s12967-019-1957-5. J Transl Med. 2019. PMID: 31221178 Free PMC article.
-
The current advances and future directions of PD-1/PD-L1 blockade in head and neck squamous cell carcinoma (HNSCC) in the era of immunotherapy.Int Immunopharmacol. 2023 Jul;120:110329. doi: 10.1016/j.intimp.2023.110329. Epub 2023 May 17. Int Immunopharmacol. 2023. PMID: 37207445 Review.
-
The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer.Front Immunol. 2020 Sep 18;11:1721. doi: 10.3389/fimmu.2020.01721. eCollection 2020. Front Immunol. 2020. PMID: 33072064 Free PMC article. Review.
Cited by
-
Radiotherapy elicits immunogenic cell death and metabolic shifts in the tumor microenvironment: implications for immunotherapy.Int J Med Sci. 2025 Jul 11;22(13):3277-3291. doi: 10.7150/ijms.109515. eCollection 2025. Int J Med Sci. 2025. PMID: 40765564 Free PMC article. Review.
-
Immune checkpoint inhibitor use in head and neck squamous cell carcinoma: the current landscape and future perspectives.Future Oncol. 2024;20(23):1695-1711. doi: 10.1080/14796694.2024.2362612. Epub 2024 Jun 18. Future Oncol. 2024. PMID: 38889284 Free PMC article. Review.
-
Immunogenic Cell Death as a Target for Combination Therapies in Solid Tumors: A Systematic Review Toward a New Paradigm in Immuno-Oncology.Cureus. 2025 Jun 11;17(6):e85776. doi: 10.7759/cureus.85776. eCollection 2025 Jun. Cureus. 2025. PMID: 40656384 Free PMC article. Review.
-
Amplifying Curcumin's Antitumor Potential: A Heat-Driven Approach for Colorectal Cancer Treatment.Onco Targets Ther. 2024 Jan 30;17:63-78. doi: 10.2147/OTT.S448024. eCollection 2024. Onco Targets Ther. 2024. PMID: 38313386 Free PMC article.
-
Abscopal effect: from a rare phenomenon to a new frontier in cancer therapy.Biomark Res. 2024 Sep 4;12(1):98. doi: 10.1186/s40364-024-00628-3. Biomark Res. 2024. PMID: 39228005 Free PMC article. Review.
References
-
- Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

