Pendrin abundance, subcellular distribution, and function are unaffected by either αENaC gene ablation or by increasing ENaC channel activity
- PMID: 36977894
- PMCID: PMC10105674
- DOI: 10.1007/s00424-023-02797-w
Pendrin abundance, subcellular distribution, and function are unaffected by either αENaC gene ablation or by increasing ENaC channel activity
Abstract
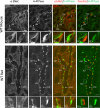
The intercalated cell Cl-/HCO3- exchanger, pendrin, modulates ENaC subunit abundance and function. Whether ENaC modulates pendrin abundance and function is however unknown. Because αENaC mRNA has been detected in pendrin-positive intercalated cells, we hypothesized that ENaC, or more specifically the αENaC subunit, modulates intercalated cell function. The purpose of this study was therefore to determine if αENaC is expressed at the protein level in pendrin-positive intercalated cells and to determine if αENaC gene ablation or constitutively upregulating ENaC activity changes pendrin abundance, subcellular distribution, and/or function. We observed diffuse, cytoplasmic αENaC label in pendrin-positive intercalated cells from both mice and rats, with much lower label intensity in pendrin-negative, type A intercalated cells. However, while αENaC gene ablation within principal and intercalated cells of the CCD reduced Cl- absorption, it did not change pendrin abundance or subcellular distribution in aldosterone-treated mice. Further experiments used a mouse model of Liddle's syndrome to explore the effect of increasing ENaC channel activity on pendrin abundance and function. The Liddle's variant did not increase either total or apical plasma membrane pendrin abundance in aldosterone-treated or in NaCl-restricted mice. Similarly, while the Liddle's mutation increased total Cl- absorption in CCDs from aldosterone-treated mice, it did not significantly affect the change in Cl- absorption seen with pendrin gene ablation. We conclude that in rats and mice, αENaC localizes to pendrin-positive ICs where its physiological role remains to be determined. While pendrin modulates ENaC abundance, subcellular distribution, and function, ENaC does not have a similar effect on pendrin.
Keywords: Aldosterone; ENaC; Intercalated cells; Pendrin.
© 2023. The Author(s).
Conflict of interest statement
S.M.W. owns stock in Johnson & Johnson, Merck & Co., Abbott Laboratories, Thermo Fisher Scientific, Becton Dickinson & Co., and Danaher. All other authors declare no competing interests.
Figures






References
-
- Bertog M, Cuffe JE, Pradervand S, Hummler E, Hartner A, Porst M, Hilgers KF, Rossier BC, Korbmacher C. Aldosterone responsiveness of the epithelial sodium channel (ENaC) in colon is increased in a mouse model for Liddle's syndrome. J Physiol. 2008;586:459–475. doi: 10.1113/jphysiol.2007.140459. - DOI - PMC - PubMed
-
- Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Paunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci U S A. 2017;114:E9989–E9998. doi: 10.1073/pnas.1710964114. - DOI - PMC - PubMed
-
- Dahlmann A, Pradervand S, Hummler E, Rossier BC, Frindt G, Palmer LG. Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle's syndrome. AmJPhysiol. 2003;285:F310–F318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

