M1 polarization enhances the antitumor activity of chimeric antigen receptor macrophages in solid tumors
- PMID: 36978075
- PMCID: PMC10044396
- DOI: 10.1186/s12967-023-04061-2
M1 polarization enhances the antitumor activity of chimeric antigen receptor macrophages in solid tumors
Abstract
Background: Chimeric antigen receptor macrophage (CAR-M) therapy is a novel cancer immunotherapy approach that integrates CAR structure and macrophage functions. CAR-M therapy has shown unique and impressive antitumor effects in immunotherapy for solid tumors. However, the polarization state of macrophages can affect the antitumor effect of CAR-M. We hypothesized that the antitumor activity of CAR-Ms may be further improved after inducing M1-type polarization.
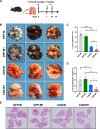
Methods: In this report, we constructed a novel HER2-targeting CAR-M, which was composed of humanized anti-HER2 scFv, CD28 hinge region and FcγRI transmembrane domain and intracellular domain. Phagocytosis, tumor-killing capacities, and cytokine release of CAR-Ms were detected with or without M1-polarization pretreatment. Several syngeneic tumor models were used to monitor the in vivo antitumor activity of M1-polarized CAR-Ms.
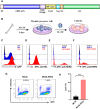
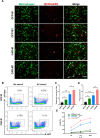
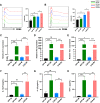
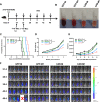
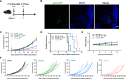
Results: After polarization with LPS combined with interferon-γ in vitro, we found that the phagocytic and tumor-killing capacities of CAR-Ms against target cells were significantly enhanced. The expression of costimulatory molecules and proinflammatory cytokines was also significantly increased after polarization. By establishing several syngeneic tumor models in vivo, we also demonstrated that infusing polarized M1-type CAR-Ms could effectively suppress tumor progression and prolong the survival of tumor-bearing mice with enhanced cytotoxicity.
Conclusions: We demonstrated that our novel CAR-M can effectively eliminate HER2-positive tumor cells both in vitro and in vivo, and M1 polarization significantly enhanced the antitumor ability of CAR-M, resulting in a stronger therapeutic effect in solid cancer immunotherapy.
Keywords: CAR-M; Cancer immunotherapy; Chimeric antigen receptor; HER2; M1 polarization; Macrophages.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

