Low-Level Tetracycline Resistance Gene tet(O) _3 in Campylobacter jejuni
- PMID: 36978293
- PMCID: PMC10044288
- DOI: 10.3390/antibiotics12030426
Low-Level Tetracycline Resistance Gene tet(O) _3 in Campylobacter jejuni
Abstract
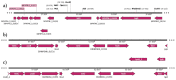
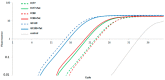
Campylobacter (C.) spp. are the most important foodborne, bacterial, and zoonotic pathogens worldwide. Resistance monitoring of foodborne bacterial pathogens is an important tool to control antimicrobial resistance as a part of the "One Health" approach. The detection and functionality of new resistance genes are of paramount importance in applying more effective screening methods based on whole genome sequencing (WGS). Most tetracycline-resistant C. spp. isolates harbor tet(O), a gene that encodes a ribosomal protection protein. Here we describe tet(O)_3, which has been identified in two food isolates of C. jejuni and is very similar to the tet(O) gene in Streptococcus pneumoniae, having a truncated promoter sequence. This gene confers resistance to tetracycline below 1 mg/L, which is the epidemiological cut-off value. We have analyzed the entire genome of these two isolates, together with a C. jejuni isolate found to have high-level resistance to tetracycline. In contrast to the highly resistant isolate, the promoter of tet(O)_3 is highly responsive to tetracycline, as observed by reverse transcription polymerase chain reaction (RT-PCR). In addition, the two isolates possess a CRISPR repeat, fluoroquinolone resistance due to the gyrA point mutation C257T, a β-lactamase resistance gene blaOXA-184, a multidrug efflux pump CmeABC and its repressor CmeR, but no plasmid. Low-level antibiotic resistant C. jejuni might therefore have an advantage for surviving in non-host environments.
Keywords: epidemiological cutoff; foodborne pathogen; multidrug efflux pump; tetracycline resistance; β-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rossler E., Signorini M.L., Romero-Scharpen A., Soto L.P., Berisvil A., Zimmermann J.A., Fusari M.L., Olivero C., Zbrun M.V., Frizzo L.S. Meta-analysis of the prevalence of thermotolerant Campylobacter in food-producing animals worldwide. Zoonoses Public Health. 2019;66:359–369. doi: 10.1111/zph.12558. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

