Effect of Antibiotic Eye Drops on the Nasal Microbiome in Healthy Subjects-A Pilot Study
- PMID: 36978384
- PMCID: PMC10044076
- DOI: 10.3390/antibiotics12030517
Effect of Antibiotic Eye Drops on the Nasal Microbiome in Healthy Subjects-A Pilot Study
Abstract
Background: Antibiotic eye drops are frequently used in clinical practice. Due to the anatomical connection via the nasolacrimal duct, it seems possible that they have an influence on the nasal/pharyngeal microbiome. This was investigated by using two different commonly used antibiotic eye drops.
Methods: 20 subjects were randomized to four groups of five subjects receiving eye drops containing gentamicin, ciprofloxacin, or, as controls, unpreserved povidone or benzalkonium chloride-preserved povidone. Nasal and pharyngeal swabs were performed before and after the instillation period. Swabs were analyzed by Illumina next-generation sequencing (NGS)-based 16S rRNA analysis. Bacterial culture was performed on solid media, and bacterial isolates were identified to the species level by MALDI-TOF MS. Species-dependent antimicrobial susceptibility testing was performed using single isolates and pools of isolates.
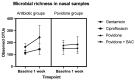
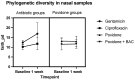
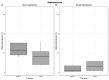
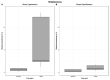
Results: Bacterial richness in the nose increased numerically from 163 ± 30 to 243 ± 100 OTUs (gentamicin) and from 114 ± 17 to 144 ± 45 OTUs (ciprofloxacin). Phylogenetic diversity index (pd) of different bacterial strains in the nasal microbiome increased from 12.4 ± 1.0 to 16.9 ± 5.6 pd (gentamicin) and from 10.2 ± 1.4 to 11.8 ± 3.1 pd (ciprofloxacin). Unpreserved povidone eye drops resulted in minimal changes in bacterial counts. Preservative-containing povidone eye drops resulted in no change. A minor increase (1-2-fold) in the minimal inhibitory concentration (MIC) was observed in single streptococcal isolates.
Conclusions: Antibiotic eye drops could affect the nasal microbiome. After an instillation period of seven days, an increase in the diversity and richness of bacterial strains in the nasal microbiome was observed.
Keywords: antibiotic eye drops; ciprofloxacin; gentamicin; nasal microbiome; next-generation sequencing.
Conflict of interest statement
None to declare. The authors declare no competing interests as well.
Figures
Similar articles
-
Bacterial microbiome in the nose of healthy cats and in cats with nasal disease.PLoS One. 2017 Jun 29;12(6):e0180299. doi: 10.1371/journal.pone.0180299. eCollection 2017. PLoS One. 2017. PMID: 28662139 Free PMC article.
-
Effectiveness of 0.66% Povidone-Iodine Eye Drops on Ocular Surface Flora before Cataract Surgery: A Nationwide Microbiological Study.J Clin Med. 2021 May 19;10(10):2198. doi: 10.3390/jcm10102198. J Clin Med. 2021. PMID: 34069600 Free PMC article.
-
Disturbances in the ocular surface microbiome by perioperative antimicrobial eye drops.Front Cell Infect Microbiol. 2023 Apr 12;13:1172345. doi: 10.3389/fcimb.2023.1172345. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37124044 Free PMC article.
-
Efficacy and safety evaluation of benzalkonium chloride preserved eye-drops compared with alternatively preserved and preservative-free eye-drops in the treatment of glaucoma: a systematic review and meta-analysis.Br J Ophthalmol. 2020 Nov;104(11):1512-1518. doi: 10.1136/bjophthalmol-2019-315623. Epub 2020 Feb 12. Br J Ophthalmol. 2020. PMID: 32051133
-
Next Generation Sequencing and the Microbiome of Chronic Rhinosinusitis: A Primer for Clinicians and Review of Current Research, Its Limitations, and Future Directions.Ann Otol Rhinol Laryngol. 2016 Aug;125(8):613-21. doi: 10.1177/0003489416641429. Epub 2016 Apr 7. Ann Otol Rhinol Laryngol. 2016. PMID: 27056556 Review.
References
LinkOut - more resources
Full Text Sources

