Conjugation as a Tool in Therapeutics: Role of Amino Acids/Peptides-Bioactive (Including Heterocycles) Hybrid Molecules in Treating Infectious Diseases
- PMID: 36978399
- PMCID: PMC10044335
- DOI: 10.3390/antibiotics12030532
Conjugation as a Tool in Therapeutics: Role of Amino Acids/Peptides-Bioactive (Including Heterocycles) Hybrid Molecules in Treating Infectious Diseases
Abstract
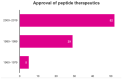
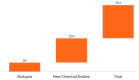
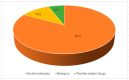
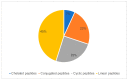
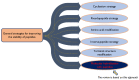
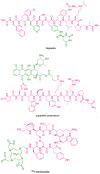
Peptide-based drugs are gaining significant momentum in the modern drug discovery, which is witnessed by the approval of new drugs by the FDA in recent years. On the other hand, small molecules-based drugs are an integral part of drug development since the past several decades. Peptide-containing drugs are placed between small molecules and the biologics. Both the peptides as well as the small molecules (mainly heterocycles) pose several drawbacks as therapeutics despite their success in curing many diseases. This gap may be bridged by utilising the so called 'conjugation chemistry', in which both the partners are linked to one another through a stable chemical bond, and the resulting conjugates are found to possess attracting benefits, thus eliminating the stigma associated with the individual partners. Over the past decades, the field of molecular hybridisation has emerged to afford us new and efficient molecular architectures that have shown high promise in medicinal chemistry. Taking advantage of this and also considering our experience in this field, we present herein a review concerning the molecules obtained by the conjugation of peptides (amino acids) to small molecules (heterocycles as well as bioactive compounds). More than 125 examples of the conjugates citing nearly 100 references published during the period 2000 to 2022 having therapeutic applications in curing infectious diseases have been covered.
Keywords: amino acids; bioactive molecules; conjugation chemistry; heterocycles; peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




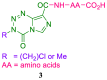
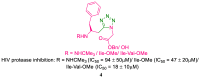


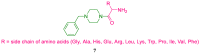
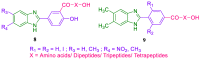










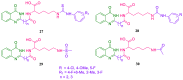
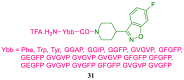

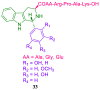
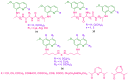

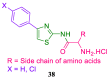


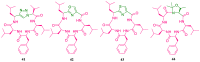
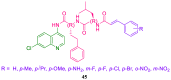
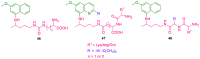






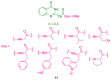
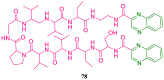
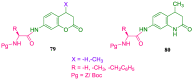

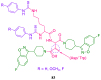
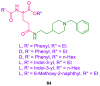
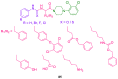
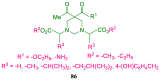


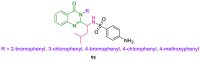
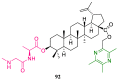
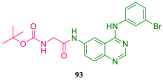

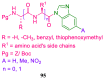














References
-
- Ceresia G., Brusch C. An introduction to the history of medicinal chemistry. Am. J. Pharm. Sci. Support. Public Health. 1955;127:384–395. - PubMed
-
- Timmerman H. Comprehensive Medicinal Chemistry II. Volume 8. Elsevier; Amsterdam, The Netherlands: 2007. Reflections on Medicinal Chemistry Since the 1950s; pp. 7–15.
-
- AACP Institutional Members. [(accessed on 15 August 2011)]. Available online: http://www.aacp.org/about/membership/institutionalmembership/Pages/usins....
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

