The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus pyogenes and Its Toxicity Assessment in Danio rerio
- PMID: 36978412
- PMCID: PMC10044342
- DOI: 10.3390/antibiotics12030545
The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus pyogenes and Its Toxicity Assessment in Danio rerio
Abstract
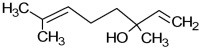
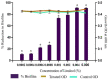
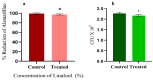
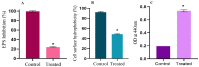
The anti-biofilm and anti-virulence potential of the essential oil (E.O.) extracted from Hedychium larsenii M. Dan & Sathish was determined against Streptococcus pyogenes. A crystal violet assay was employed to quantify the biofilm. Linalool, a monoterpene alcohol from the E.O., showed concentration-dependent biofilm inhibition, with a maximum of 91% at a concentration of 0.004% (v/v). The AlamarBlueTM assay also confirmed Linalool's non-bactericidal anti-biofilm efficacy (0.004%). Linalool treatment impeded micro-colony formation, mature biofilm architecture, surface coverage, and biofilm thickness and impaired cell surface hydrophobicity and EPS production. Cysteine protease synthesis was quantified using the Azocasein assay, and Linalool treatment augmented its production. This suggests that Linalool destabilizes the biofilm matrix. It altered the expression of core regulons covRS, mga, srv, and ropB, and genes associated with virulence and biofilm formation, such as speB, dltA, slo, hasA, and ciaH, as revealed by qPCR analysis. Cytotoxicity analysis using human kidney cells (HEK) and the histopathological analysis in Danio rerio proved Linalool to be a druggable molecule against the biofilms formed by S. pyogenes. This is the first report on Linalool's anti-biofilm and anti-virulence potential against S. pyogenes.
Keywords: AlamarBlueTM assay; Danio rerio; Hedychium larsenii; Streptococcus pyogenes; biofilm; linalool.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
5-Dodecanolide inhibits biofilm formation and virulence of Streptococcus pyogenes by suppressing core regulons of virulence.Life Sci. 2020 Dec 1;262:118554. doi: 10.1016/j.lfs.2020.118554. Epub 2020 Oct 6. Life Sci. 2020. PMID: 33035584
-
Fukugiside, a biflavonoid from Garcinia travancorica inhibits biofilm formation of Streptococcus pyogenes and its associated virulence factors.J Med Microbiol. 2018 Sep;67(9):1391-1401. doi: 10.1099/jmm.0.000799. Epub 2018 Jul 27. J Med Microbiol. 2018. PMID: 30052177
-
Carvacrol inhibits Streptococcus pyogenes biofilms by suppressing the expression of genes associated with quorum-sensing and reducing cell surface hydrophobicity.Microb Pathog. 2022 Aug;169:105684. doi: 10.1016/j.micpath.2022.105684. Epub 2022 Jul 19. Microb Pathog. 2022. PMID: 35863588
-
Application of Medicinal Plants as a Source for Therapeutic Agents Against Streptococcus pyogenes Infections.Curr Drug Metab. 2018;19(8):695-703. doi: 10.2174/1389200219666180329150551. Curr Drug Metab. 2018. PMID: 29595103 Review.
-
Streptococcus pyogenes Biofilm.2022 Aug 21 [updated 2022 Oct 4]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd edition. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 15. 2022 Aug 21 [updated 2022 Oct 4]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd edition. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 15. PMID: 36479769 Free Books & Documents. Review. No abstract available.
Cited by
-
Biofilm inhibition/eradication: exploring strategies and confronting challenges in combatting biofilm.Arch Microbiol. 2024 Apr 14;206(5):212. doi: 10.1007/s00203-024-03938-0. Arch Microbiol. 2024. PMID: 38616221 Review.
-
Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance.Antibiotics (Basel). 2025 May 13;14(5):503. doi: 10.3390/antibiotics14050503. Antibiotics (Basel). 2025. PMID: 40426569 Free PMC article. Review.
-
Linalool combats Saprolegnia parasitica infections through direct killing of microbes and modulation of host immune system.Elife. 2025 Apr 4;13:RP100393. doi: 10.7554/eLife.100393. Elife. 2025. PMID: 40183210 Free PMC article.
-
Antibiofilm Power of Basil Essential Oil Against Fish-Originated Multidrug-Resistant Salmonella and Bacillus spp.: Targeting Biofilms on Food Contact Surfaces.Foods. 2025 May 21;14(10):1830. doi: 10.3390/foods14101830. Foods. 2025. PMID: 40428609 Free PMC article.
-
Synergistic anti-biofilm strategy based on essential oils and its application in the food industry.World J Microbiol Biotechnol. 2025 Feb 27;41(3):81. doi: 10.1007/s11274-025-04289-8. World J Microbiol Biotechnol. 2025. PMID: 40011295 Review.
References
-
- Wójcik M., Eleftheriadis N., Zwinderman M.R., Dömling A.S., Dekker F.J., Boersma Y.L. Identification of potential antivirulence agents by substitution-oriented screening for inhibitors of Streptococcus pyogenes sortase A. Eur. J. Med. Chem. 2019;161:93–100. doi: 10.1016/j.ejmech.2018.10.027. - DOI - PubMed
-
- Hu H., Johani K., Gosbell I.B., Jacombs A.S., Almatroudi A., Whiteley G.S., Deva A.K., Jensen S., Vickery K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in bioflms: Combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J. Hosp. Infect. 2015;91:35–44. doi: 10.1016/j.jhin.2015.05.016. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

