Design and Synthesis of Novel Antimicrobial Agents
- PMID: 36978495
- PMCID: PMC10045396
- DOI: 10.3390/antibiotics12030628
Design and Synthesis of Novel Antimicrobial Agents
Abstract
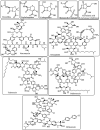
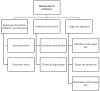
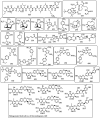
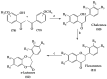
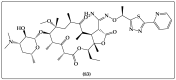
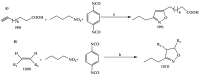
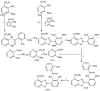
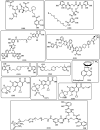
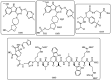
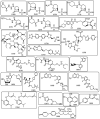
The necessity for the discovery of innovative antimicrobials to treat life-threatening diseases has increased as multidrug-resistant bacteria has spread. Due to antibiotics' availability over the counter in many nations, antibiotic resistance is linked to overuse, abuse, and misuse of these drugs. The World Health Organization (WHO) recognized 12 families of bacteria that present the greatest harm to human health, where options of antibiotic therapy are extremely limited. Therefore, this paper reviews possible new ways for the development of novel classes of antibiotics for which there is no pre-existing resistance in human bacterial pathogens. By utilizing research and technology such as nanotechnology and computational methods (such as in silico and Fragment-based drug design (FBDD)), there has been an improvement in antimicrobial actions and selectivity with target sites. Moreover, there are antibiotic alternatives, such as antimicrobial peptides, essential oils, anti-Quorum sensing agents, darobactins, vitamin B6, bacteriophages, odilorhabdins, 18β-glycyrrhetinic acid, and cannabinoids. Additionally, drug repurposing (such as with ticagrelor, mitomycin C, auranofin, pentamidine, and zidovudine) and synthesis of novel antibacterial agents (including lactones, piperidinol, sugar-based bactericides, isoxazole, carbazole, pyrimidine, and pyrazole derivatives) represent novel approaches to treating infectious diseases. Nonetheless, prodrugs (e.g., siderophores) have recently shown to be an excellent platform to design a new generation of antimicrobial agents with better efficacy against multidrug-resistant bacteria. Ultimately, to combat resistant bacteria and to stop the spread of resistant illnesses, regulations and public education regarding the use of antibiotics in hospitals and the agricultural sector should be combined with research and technological advancements.
Keywords: antibiotic; antimicrobial agents; antimicrobial peptides; nanoparticles; resistance; siderophores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















References
Publication types
LinkOut - more resources
Full Text Sources

