Reduced Fibroblast Activation on Electrospun Polycaprolactone Scaffolds
- PMID: 36978739
- PMCID: PMC10045272
- DOI: 10.3390/bioengineering10030348
Reduced Fibroblast Activation on Electrospun Polycaprolactone Scaffolds
Abstract
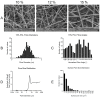
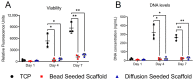
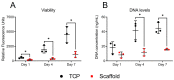
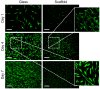
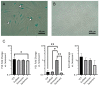
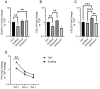
In vivo, quiescent fibroblasts reside in three-dimensional connective tissues and are activated in response to tissue injury before proliferating rapidly and becoming migratory and contractile myofibroblasts. When deregulated, chronic activation drives fibrotic disease. Fibroblasts cultured on stiff 2D surfaces display a partially activated phenotype, whilst many 3D environments limit fibroblast activation. Cell mechanotransduction, spreading, polarity, and integrin expression are controlled by material mechanical properties and micro-architecture. Between 3D culture systems, these features are highly variable, and the challenge of controlling individual properties without altering others has led to an inconsistent picture of fibroblast behaviour. Electrospinning offers greater control of mechanical properties and microarchitecture making it a valuable model to study fibroblast activation behaviour in vitro. Here, we present a comprehensive characterisation of the activation traits of human oral fibroblasts grown on a microfibrous scaffold composed of electrospun polycaprolactone. After over 7 days in the culture, we observed a reduction in proliferation rates compared to cells cultured in 2D, with low KI67 expression and no evidence of cellular senescence. A-SMA mRNA levels fell, and the expression of ECM protein-coding genes also decreased. Electrospun fibrous scaffolds, therefore, represent a tuneable platform to investigate the mechanisms of fibroblast activation and their roles in fibrotic disease.
Keywords: 3D cell culture; biomaterials; electrospinning; fibroblast; scaffold.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

